TRANSCRIBER'S NOTE
Blank space in some sample documents in the text is denoted by ____.
Footnotes are all within a specific Table, including the Table of Contents. They are positioned at the bottom of that Table, as in the original text, and are denoted by [*] or [**].
Page numbering of the original text has been retained. It is in the form a-b, where a is a Chapter number or Appendix letter, and b is the sequential number within that section. For example B-3 is the third page in Appendix B.
Obvious punctuation errors have been corrected after careful comparison with other occurrences within the text and consultation of external sources.
The cover image was created by the transcriber
and is placed in the public domain.
More detail can be found at the end of the book.
FM 4-02.7 (FM 8-10-7)
TACTICS, TECHNIQUES, AND PROCEDURES
OCTOBER 2002
HEADQUARTERS, DEPARTMENT OF THE ARMY
DISTRIBUTION RESTRICTION: Approved for public release; distribution is unlimited.
[Pg i] [*]FM 4-02.7 (FM 8-10-7)
FIELD MANUAL
HEADQUARTERS
NO. 4-02.7 (8-10-7)
DEPARTMENT OF THE ARMY
Washington, DC, 1 October 2002
HEALTH SERVICE SUPPORT IN A NUCLEAR, BIOLOGICAL,
AND CHEMICAL ENVIRONMENT
TACTICS, TECHNIQUES AND PROCEDURES
| Page | |||
| Preface | viii | ||
| CHAPTER | 1. | NUCLEAR, BIOLOGICAL, AND CHEMICAL WARFARE ASPECT OF THE MEDICAL THREAT | 1-1 |
| 1-1. | General | 1-1 | |
| 1-2. | Medical Threat | 1-1 | |
| 1-3. | Nuclear, Biological, Chemical, and Radiological Dispersal Device Threats—The Health Service Perspective | 1-2 | |
| CHAPTER | 2. | COMMAND AND CONTROL | 2-1 |
| 2-1. | General | 2-1 | |
| 2-2. | Health Service Support Command and Control Planning Considerations | 2-1 | |
| 2-3. | Health Service Support Command and Control Appraisal of the Support Mission | 2-2 | |
| 2-4. | Health Service Support Units | 2-2 | |
| 2-5. | Movement/Management of Contaminated Facilities | 2-3 | |
| 2-6. | Leadership on the Contaminated Battlefield | 2-5 | |
| 2-7. | Homeland Security | 2-6 | |
| CHAPTER | 3. | LEVELS I AND II HEALTH SERVICE SUPPORT | 3-1 |
| 3-1. | General | 3-1 | |
| 3-2. | Level I Health Service Support | 3-2 | |
| 3-3. | Level II Health Service Support | 3-2 | |
| 3-4. | Forward Surgical Team | 3-3 | |
| 3-5. | Actions Before a Nuclear, Biological, or Chemical Attack | 3-3 | |
| 3-6. | Actions During a Nuclear, Biological, or Chemical Attack | 3-4 | |
| 3-7. | Actions After a Nuclear, Biological, or Chemical Attack | 3-4 | |
| 3-8. | Logistical Considerations | 3-5 | |
| [ii] 3-9. | Personnel Considerations | 3-5 | |
| 3-10. | Disposition and Employment of Treatment Elements | 3-6 | |
| 3-11. | Civilian Casualties | 3-6 | |
| 3-12. | Nuclear Environment | 3-7 | |
| 3-13. | Medical Triage | 3-8 | |
| 3-14. | Biological Environment | 3-8 | |
| 3-15. | Chemical Environment | 3-9 | |
| 3-16. | Operations in Extreme Environments | 3-10 | |
| 3-17. | Medical Evacuation in a Nuclear, Biological, and Chemical Environment | 3-10 | |
| CHAPTER | 4. | LEVELS III AND IV HOSPITALIZATION | 4-1 |
| 4-1. | General | 4-1 | |
| 4-2. | Protection | 4-3 | |
| 4-3. | Decontamination | 4-8 | |
| 4-4. | Emergency Services | 4-10 | |
| 4-5. | General Medical Services | 4-11 | |
| 4-6. | Surgical Services | 4-11 | |
| 4-7. | Nursing Services | 4-12 | |
| 4-8. | Conventional Operations | 4-13 | |
| CHAPTER | 5. | OTHER HEALTH SERVICE SUPPORT | 5-1 |
| Section | I. | Preventive Medicine Services | 5-1 |
| 5-1. | General | 5-1 | |
| 5-2. | Disease Incidence Following the Use of Nuclear, Biological, and Chemical Weapons | 5-1 | |
| 5-3. | Preventive Medicine Section | 5-3 | |
| 5-4. | Preventive Medicine Detachment | 5-3 | |
| Section | II. | Veterinary Services | 5-4 |
| 5-5. | General | 5-4 | |
| 5-6. | Food Protection | 5-4 | |
| 5-7. | Food Decontamination | 5-4 | |
| 5-8. | Animal Care | 5-5 | |
| Section | III. | Laboratory Services | 5-5 |
| 5-9. | General | 5-5 | |
| 5-10. | Level II | 5-5 | |
| 5-11. | Level III | 5-5 | |
| 5-12. | Level IV | 5-6 | |
| 5-13. | Level V (Continental United States) | 5-6 | |
| 5-14. | Field Samples | 5-6 | |
| Section | IV. | Dental Services | 5-7 |
| 5-15. | General | 5-7 | |
| 5-16. | Mission in a Nuclear, Biological, or Chemical Environment | 5-7 | |
| [iii] 5-17. | Dental Treatment Operations | 5-7 | |
| 5-18. | Patient Treatment Considerations | 5-7 | |
| 5-19. | Patient Protection | 5-8 | |
| Section | V. | Combat Operational Stress Control | 5-9 |
| 5-20. | General | 5-9 | |
| 5-21. | Leadership Actions | 5-9 | |
| 5-22. | Individual Responsibilities | 5-10 | |
| 5-23. | Mental Health Personnel Responsibilities | 5-11 | |
| Section | VI. | Health Service Logistics | 5-11 |
| 5-24. | General | 5-11 | |
| 5-25. | Protecting Supplies In Storage | 5-12 | |
| 5-26. | Protecting Supplies During Shipment | 5-12 | |
| 5-27. | Organizational Maintenance | 5-12 | |
| Section | VII. | Homeland Security Response | 5-13 |
| 5-28. | Chemical, Biological, Radiological, Nuclear, and High-Yield Explosive Response | 5-13 | |
| 5-29. | Capabilities of Response Elements | 5-14 | |
| APPENDIX | A. | MEDICAL EFFECTS OF NUCLEAR, BIOLOGICAL, AND CHEMICAL WEAPONS AND TOXIC INDUSTRIAL MATERIAL | A-1 |
| A-1. | General | A-1 | |
| A-2. | Physical Effects of Nuclear Weapons | A-1 | |
| A-3. | Physiological Effects of Nuclear Weapons | A-4 | |
| A-4. | Biological Effects of Thermal Radiation | A-7 | |
| A-5. | Physiological Effects of Ionizing Radiation | A-8 | |
| A-6. | Handling and Managing Radiologically Contaminated Patients | A-10 | |
| A-7. | Radiological Patients in Stability Operations and Support Operations | A-13 | |
| A-8. | Effects of Biological Weapons | A-14 | |
| A-9. | Behavior of Biological Weapons | A-15 | |
| A-10. | Management of Biological Warfare Patients | A-16 | |
| A-11. | Effects of Chemical Weapons | A-17 | |
| A-12. | Behavior of Chemical Weapons | A-17 | |
| A-13. | Characteristics of Chemical Agents | A-19 | |
| A-14. | Management of Chemical Agent Patients | A-23 | |
| A-15. | Management of Toxic Industrial Material Patients | A-23 | |
| APPENDIX | B. | SAMPLE/SPECIMEN COLLECTION AND MANAGEMENT | B-1 |
| Section | I. | Introduction | B-1 |
| B-1. | General | B-1 | |
| B-2. | Sample/Specimen Background Information | B-2 | |
| B-3. | Sample/Specimen Collection and Preservation | B-3 | |
| B-4. | Chain of Custody | B-8 | |
| [iv] Section | II. | Sampling Techniques and Procedures | B-9 |
| B-5. | General | B-9 | |
| B-6. | Expended Material | B-11 | |
| B-7. | Environmental Samples | B-11 | |
| B-8. | Collection of Air and Vapors | B-12 | |
| B-9. | Collection of Water Samples | B-13 | |
| B-10. | Collection of Soil Samples | B-15 | |
| B-11. | Collection of Contaminated Vegetation | B-16 | |
| B-12. | Medical Specimens | B-16 | |
| B-13. | Collection of Medical Specimens | B-17 | |
| B-14. | Post Mortem Specimens | B-19 | |
| B-15. | Reporting, Packaging, and Shipment | B-20 | |
| B-16. | Handling and Packaging Materials | B-21 | |
| B-17. | Collection Reporting | B-23 | |
| B-18. | Sample/Specimen Background Documents | B-27 | |
| APPENDIX | C. | GUIDELINES FOR OPERATIONAL PLANNING FOR HEALTH SERVICE SUPPORT IN A NUCLEAR, BIOLOGICAL, AND CHEMICAL ENVIRONMENT | C-1 |
| C-1. | General | C-1 | |
| C-2. | Predeployment | C-1 | |
| C-3. | Mobilization | C-2 | |
| C-4. | Establish a Medical Treatment Facility | C-3 | |
| C-5. | Operate a Medical Treatment Facility Receiving Contaminated Patients. | C-4 | |
| C-6. | Preventive Medicine Services | C-5 | |
| C-7. | Veterinary Services | C-6 | |
| C-8. | Dental Services | C-6 | |
| C-9. | Combat Operational Stress Control | C-6 | |
| C-10. | Medical Laboratory Services | C-6 | |
| C-11. | Health Service Logistics | C-7 | |
| C-12. | Homeland Security | C-8 | |
| APPENDIX | D. | MEDICAL PLANNING GUIDE FOR THE ESTIMATION OF NUCLEAR, BIOLOGICAL, AND CHEMICAL BATTLE CASUALTIES | D-1 |
| Section | I. | Introduction | D-1 |
| D-1. | General | D-1 | |
| D-2. | Medical Planners' Tool | D-1 | |
| Section | II. | Medical Planning Guide for the Estimation of Nuclear, Biological, and Chemical Battle Casualties (Nuclear)—AMedP-8(A), Volume I | D-1 |
| D-3. | General | D-1 | |
| D-4. | Medical Planning Considerations | D-2 | |
| [v] D-5. | Triage | D-3 | |
| D-6. | Evacuation | D-3 | |
| D-7. | In-Unit Care | D-3 | |
| D-8. | Hospital Bed Requirements | D-4 | |
| D-9. | Medical Logistics | D-4 | |
| D-10. | Medical Force Planning | D-4 | |
| Section | III. | Medical Planning Guide for the Estimation of Nuclear, Biological, and Chemical Battle Casualties (Biological)—AMedP-8(A), Volume II | D-4 |
| D-11. | General | D-4 | |
| D-12. | Medical Planning Considerations | D-6 | |
| D-13. | Triage | D-6 | |
| D-14. | Evacuation | D-6 | |
| D-15. | In-Unit Care | D-7 | |
| D-16. | Patient Bed Requirements | D-7 | |
| D-17. | Medical Logistics | D-7 | |
| D-18. | Medical Force Planning | D-8 | |
| Section | IV. | Medical Planning Guide for the Estimation of Nuclear, Biological, and Chemical Battle Casualties (Chemical)—AMedP-8(A), Volume III | D-8 |
| D-19. | General | D-8 | |
| D-20. | Medical Planning Considerations | D-10 | |
| D-21. | Triage | D-11 | |
| D-22. | Evacuation | D-11 | |
| D-23. | In-Unit Care | D-11 | |
| D-24. | Patient Bed Requirements | D-12 | |
| D-25. | Medical Logistics | D-12 | |
| D-26. | Medical Force Planning | D-12 | |
| APPENDIX | E. | EXAMPLE X-__, ANNEX__, TO HSS PLAN/OPERATION ORDER__, MEDICAL NBC STAFF OFFICER PLANNING FOR HSS IN AN NBC ENVIRONMENT | E-1 |
| APPENDIX | F. | EMPLOYMENT OF CHEMICAL AND BIOLOGICAL COLLECTIVE PROTECTION SHELTER SYSTEMS BY MEDICAL UNITS | F-1 |
| Section | I. | Introduction | F-1 |
| F-1. | General | F-1 | |
| F-2. | Types of Collective Protection Shelter Systems | F-1 | |
| Section | II. | Employment of the Chemically and Biologically Protected Shelter System | F-2 |
| F-3. | Establish a Battalion Aid Station in a Chemically Biologically Protected Shelter | F-2 | |
| [vi] F-4. | Division Clearing Station in a Chemically Biologically Protected Shelter | F-4 | |
| F-5. | Forward Surgical Team in a Chemically Biologically Protected Shelter | F-6 | |
| Section | III. | Employment of the Chemically Protected Deployable Medical Systems and Simplified Collective Protection Systems | F-8 |
| F-6. | Collective Protection in a Deployable Medical System-Equipped Hospital | F-8 | |
| F-7. | Chemically/Biologically Protecting the International Organization for Standardization Shelter | F-11 | |
| F-8. | Chemically/Biologically Protecting the Vestibules | F-12 | |
| F-9. | Chemically/Biologically Protecting Air Handler Equipment | F-12 | |
| F-10. | Establish Collective Protection Shelter Using the M20 Simplified Collective Protection System | F-12 | |
| F-11. | Casualty Decontamination | F-12 | |
| Section | IV. | Operations, Entry, and Exit Guidelines | F-13 |
| F-12. | Operations | F-13 | |
| F-13. | Decontamination of Entrance Area | F-13 | |
| F-14. | Procedures Prior to Entry | F-14 | |
| F-15. | Entry/Exit for the Collective Protection Shelter System | F-14 | |
| F-16. | Resupply of Protected Areas | F-17 | |
| APPENDIX | G. | PATIENT DECONTAMINATION | G-1 |
| Section | I. | Introduction | G-1 |
| G-1. | General | G-1 | |
| G-2. | Immediate Decontamination | G-2 | |
| G-3. | Patient Decontamination and Thorough Decontamination Collocation | G-2 | |
| G-4. | Patient Decontamination at the Battalion Aid Station (Level I) | G-5 | |
| G-5. | Patient Decontamination at the Medical Company Clearing Station (Level II) | G-5 | |
| G-6. | Patient Decontamination at a Hospital (Level III and IV) | G-5 | |
| G-7. | Prepare Hypochlorite Solutions for Patient Decontamination | G-5 | |
| G-8. | Classification of Patients | G-6 | |
| G-9. | Patient Treatment | G-6 | |
| Section | II. | Patient Decontamination Procedures | G-7 |
| G-10. | Decontaminate a Litter Chemical Agent Patient | G-7 | |
| G-11. | Decontaminate an Ambulatory Chemical Agent Patient | G-14 | |
| G-12. | Biological Patient Decontamination Procedures | G-18 | |
| G-13. | Decontaminate a Litter Biological Agent Patient | G-18 | |
| G-14. | Decontaminate an Ambulatory Biological Agent Patient | G-19 | |
| G-15. | Decontaminate Nuclear-Contaminated Patients | G-20 | |
| G-16. | Decontaminate a Litter Nuclear-Contaminated Patient | G-21 | |
| G-17. | Decontaminate an Ambulatory Nuclear-Contaminated Patient | G-21 | |
| [vii] APPENDIX | H. | FIELD EXPEDIENT PROTECTIVE SYSTEMS AGAINST NUCLEAR, BIOLOGICAL, AND CHEMICAL ATTACK | H-1 |
| H-1. | General | H-1 | |
| H-2. | Protection Against Radiation | H-1 | |
| H-3. | Expedient Shelters for Protection Against Radiation | H-2 | |
| H-4. | Expedient Shelters Against Biological and Chemical Agents | H-5 | |
| APPENDIX | I. | DETECTION AND TREATMENT OF NUCLEAR, BIOLOGICAL, AND CHEMICAL CONTAMINATION IN WATER | I-1 |
| I-1. | General | I-1 | |
| I-2. | Detection of Contamination in Water | I-1 | |
| I-3. | Procedures on Discovery of Contamination in Water | I-1 | |
| I-4. | Treatment of Contaminated Water | I-2 | |
| APPENDIX | J. | FOOD CONTAMINATION AND DECONTAMINATION | J-1 |
| J-1. | General | J-1 | |
| J-2. | Protection of Food From Contamination | J-2 | |
| J-3. | Nuclear | J-3 | |
| J-4. | Biological | J-4 | |
| J-5. | Chemical | J-5 | |
| GLOSSARY | Glossary-1 | ||
| REFERENCES | References-1 | ||
| INDEX | Index-1 | ||
DISTRIBUTION RESTRICTION: Approved for public release; distribution is unlimited.
[*] This publication supersedes FM 8-10-7, 22 April 1993. Change 1, 26 November 1996
The purpose of this field manual (FM) is to provide doctrine and tactics, techniques, and procedures for health service support (HSS) units and personnel operating in a nuclear, biological, and chemical (NBC), radiological dispersal device (RDD), and toxic industrial material (TIM) environment. The manual provides information for use by commanders, planners, leaders, and individuals in providing HSS under these adverse conditions.
The use of trade or brand names in this publication is for illustrative purposes only. Their use does not constitute endorsement by the Department of Defense (DOD).
The proponent of this publication is the United States (US) Army Medical Department Center and School (AMEDDC&S). Send comments and recommendations directly to Commander, US Army Medical Department Center and School, ATTN: MCCS-FCD, 1400 East Grayson Street, Fort Sam Houston, Texas 78234-5052.
The use of the term "level of care" in this publication is synonymous with "echelon of care" and "role of care." The term "echelon of care" is the old North Atlantic Treaty Organization (NATO) term. The term "role of care" is the new NATO and American, British, Canadian, and Australian (ABCA) term.
The use of the term TIM in this publication is inclusive of RDD.
The use of the term "Health Service Support" in this publication is synonymous with Combat Health Support as used in other publications. Health Service Support is the term used in Joint Publications to describe medical support to Joint Forces.
Radiological and chemical detection devices discussed in this publication are currently being replaced through modernization or new device developments. The users should adapt the application of doctrine as described to fit the new devices when issued/authorized.
Unless this publication states otherwise, masculine nouns and pronouns do not refer exclusively to men.
This publication implements NATO Standardization Agreements (STANAGs) 2475, Medical Planning Guide for the Estimation of NBC Battle Casualties (Nuclear)—Allied Medical Publication (AMedP) 8(A), Volume I; 2476, Medical Planning Guide of NBC Battle Casualties (Biological)—AMedP-8(A), Volume II; 2477, Planning Guide for the Estimation of NBC Battle Casualties (Chemical)—AMedP-8 (A), Volume III. It is also in consonance with the following NATO STANAGs and ABCA Quadripartite Standardization Agreements (QSTAGs):
| TITLE | STANAG | QSTAG |
| Warning Signs for the Marking of Contaminated or Dangerous Land Areas, Complete Equipments, Supplies and Stores | 2002 | 501 |
| Emergency Alarms of Hazard or Attack (NBC and Air Attack Only) | 2047 | 183 |
| [ix]Interoperable Chemical Agent Detector Kits | 608 | |
| Emergency War Surgery | 2068 | |
| Commander's Guide on Nuclear Radiation Exposure of Groups | 2083 | 898 |
| Reporting Nuclear Detonations, Biological and Chemical Attacks, and Predicting and Warning of Associated Hazards and Hazard Areas—ATP-45(B) | 2103 | 187 |
| Friendly Nuclear Strike Warning | 2104 | 189 |
| Nuclear, Biological and Chemical Reconnaissance | 2112 | |
| NATO Handbook on the Medical Aspects of NBC Defensive Operations—AMedP-6(B) | 2500 | |
| Concept of Operations of Medical Support in Nuclear, Biological, and Chemical Environments—AMedP-7(A) | 2873 | |
| Medical Aspects of NBC Defensive Operations | 1330 | |
| Principles of Medical Policy in the Management of a Mass Casualty Situation | 2879 | |
| Medical Aspects of Mass Casualty Situations | 816 | |
| Guidelines for Air and Ground Personnel Using Fixed and Transportable Collective Protection Facilities on Land | 2941 | 2000 |
| Training of Medical Personnel for NBC Operations | 2954 |
a. After World War II, the Soviet Union represented the principal threat to the national security interests of the US. During this period, the military capability of the Soviet Armed Forces grew enormously. Starting in the later years of the 1980s, the international security environment has undergone rapid, fundamental, and revolutionary changes. With the collapse of Soviet communism, the Soviet Union disintegrated as a viable economic and political system. The Warsaw Pact dissolved as a political and military entity. The central Soviet government was replaced by the Commonwealth of Independent States (CIS), dominated by the Russian Republic. The cohesion of Soviet strategic military capability has been fractured by—
The ultimate outcome of these events in terms of US national security interests is unclear. The military capabilities of CIS like Russia, Ukraine, Kazakstan, and Belarus remain formidable. The capabilities include strategic nuclear and impressive conventional, biological, and chemical warfighting capabilities.
b. From a global perspective, the economic power and influence of developing and newly industrialized nations continue to grow. Centers of power (global or regional) cannot be measured solely in military terms. Nation states pursuing their own political, ideological, and economic interests may become engaged in direct or indirect competition and conflict with the US. More nations have acquired significant numbers of modern, lethal, combat weapon systems; developed very capable armed forces; and become more assertive in international affairs. In the absence of a single, credible, coercive threat, old rivalries and long repressed territorial ambitions will resurface, causing increased tensions in many regions. Political, economic, and social instability and religious, cultural, and economic competition will continue to erode the influence of the US over the rest of the world. This erosion will also reduce the US influence of traditional regional powers over their neighbors. This environment will encourage the continued development, or acquisition, of modern armed forces and equipment by less influential nations; thus raising the potential for the use of NBC/RDD weapons during internal conflict and armed confrontations in developing regions of the world.
c. A third dimension to the threat is terrorist, rogue groups, and belligerents employing a number of chemical and biological agents and the possible use of TIM to injure or kill US personnel. The actions may be isolated or may be imposed by groups of individuals. Most will have the financial backing of nations, large organizations, or groups that have the desire to cause harm and create public distrust in our government.
Medical threat is the composite of all ongoing or potential enemy actions and environmental conditions that [1-2] will reduce combat effectiveness through wounding, injuring, causing disease, and/or degrading performance. Soldiers are the targets of these threats. Weapons or environmental conditions that will generate wounded, injured, and sick soldiers, beyond the capability of the HSS system to provide timely medical care from available resources, are considered major medical threats. Weapons or environmental conditions that produce qualitatively different wound or disease processes are also major medical threats. Added to the combat operational and disease and nonbattle injury (DNBI) medical threats are adversary use of the following types of weapons, agents, and devices:
a. Nuclear Weapons and Radiological Dispersal Device Threats. Since the breakup of the Soviet Union, the number of countries with known nuclear capable military forces has almost doubled. Available information suggests that a number of countries in the Middle East, Asia, and Africa have or may have nuclear weapons capability within the next decade. Table 1-1 lists those countries known to have, suspected of possessing, or seeking, nuclear weapons. Planners can expect, as a minimum, 10 to 20 percent casualties within a division-sized force that has experienced a nuclear strike. In addition to the casualties, a nuclear weapon detonation can generate an electromagnetic pulse (EMP) that will cause catastrophic failures of electronic equipment components. Radiological dispersal devices, comprised of an explosive device with radioactive material, can be detonated without the need for the components of a nuclear weapon. The RDD can disperse radioactive material over an area of the battlefield causing effects from nuisance levels of radioactive material to life-threatening levels without the thermal and, in most cases, the blast effects of a nuclear detonation. For nuclear weapons effects see Appendix A.
[1-3] Table 1-1. Countries Possessing or Suspected of Possessing Nuclear Weapons
| KNOWN TO POSSESS | SUSPECT OR SEEKING |
|---|---|
| UNITED STATES OF AMERICA | IRAQ |
| RUSSIA | NORTH KOREA |
| UKRAINE | IRAN |
| BELARUS | LIBYA |
| KAZAKSTAN | ALGERIA |
| PEOPLE'S REPUBLIC OF CHINA | SOUTH AFRICA |
| FRANCE | ISRAEL |
| UNITED KINGDOM | |
| PAKISTAN | |
| INDIA |
b. Biological Warfare.
(1) Biological warfare (BW) is defined by the US intelligence community as the intentional use of disease-causing organisms (pathogens), toxins, or other agents of biological origin (ABO) to incapacitate, injure, or kill humans and animals; to destroy crops; to weaken resistance to attack; and to reduce the will to fight. Historically, BW has primarily involved the use of pathogens in assassinations or as sabotage agents in food and water supplies to spread contagious disease among target populations.
(2) For purposes of medical threat risk assessment, we are interested only in those BW agents that incapacitate, injure, or kill humans or animals.
(3) Known or suspect BW agents and ABOs can generally be categorized as naturally occurring, unmodified infectious agents (pathogens); toxins, venoms, and their biologically active fractions; modified infectious agents; and bioregulators. See Table 1-2 for examples of known or suspected BW threat agents. Also, Table 1-3 presents possible developmental and future BW agents.
Table 1-2. Examples of Known or Suspect Biological Warfare Agents
| PATHOGENS | TOXINS |
|---|---|
| BACILLUS ANTHRACIS (ANTHRAX) | BOTULINUM TOXIN |
| FRANCISELLA TULARENIUS (TULAREMIA) | MYCOTOXINS |
| YERSINIA PESTIS (PLAGUE) | ENTEROTOXIN |
| BRUCELLA SPECIES (BRUCELLOSIS) | RICIN |
| VIBRIO CHOLERAE (CHOLERA) | |
| VARIOLA (SMALLPOX) | |
| VIRAL HEMORRHAGIC FEVERS |
[1-4] Table 1-3. The Future of Biological Warfare Agents
| CURRENT THREAT | FUTURE |
|---|---|
| PATHOGENS | MODIFIED PATHOGENS |
| LIMITED NUMBER OF TOXINS | EXPANDED RANGE OF TOXINS (ORGANO-TOXINS) |
| AGENTS OF BIOLOGICAL ORIGIN | PROTEIN FRACTIONS |
| AGENTS OF BIOLOGICAL ORIGIN |
(4) Many governments recognize the industrial and economic potential of advanced biotechnology and bioengineering. The same knowledge, skills, and methodologies can be applied to the production of second and third generation BW agents. Naturally occurring infectious organisms can be made more virulent and antibiotic resistant and manipulated to render protective vaccines ineffective. These developments complicate the ability to detect and identify BW agents and to operate in areas contaminated by the BW agents. For biological agent characteristics and effects see Appendix A. The first indication that a BW agent release/attack has occurred may be patients presenting at a medical treatment facility with symptoms not fitting the mold for endemic diseases in the area of operations (AO). See Appendix B for sampling requirements, sampling procedures, packaging and shipping, and chain of custody requirements.
c. Chemical Warfare.
(1) Since World War I, most western political and military leaders have publicly held chemical warfare (CW) in disrepute. However, evidence accumulated over the last 50 years does not support the position that public condemnation equates to limiting development or use of offensive CW agents. The reported use of chemical agents and biological toxins in Southeast Asia by Vietnamese forces; the confirmed use of CW agents by Egypt against Yemen; and later by Iraq against Iranian forces; and the probable use of CW agents by the Soviets in Afghanistan indicate a heightened interest in CW as a force multiplier. Also, an offensive CW capability is developed as a deterrent to the military advantage of a potential adversary. For a list of common chemical agents, their characteristics, behavior, and effects see Appendix A. Table 1-4 lists those countries known or suspected of having offensive chemical weapons.
(2) The Russian Republic has the most extensive CW capability in Europe. Chemical strikes can be delivered with almost any type of conventional fire support weapon system (from mortars to long-range tactical missiles). Agents known to be available in the Russian inventory include nerve agents (O-ethyl methyl phosphonothiolate [VX], thickened VX, Sarin [GB], and thickened Soman [GD]); vesicants (thickened Lewisite[L] and mustard-Lewisite mixture[HL]); and choking agent (phosgene). Although not considered CW agents, riot control agents are also in the Russian inventory.
(3) The US is in the process of destroying its stockpiles of CW weapons. Many weapons have already been destroyed and the storage facilities have been rendered safe of all CW agent residues.
[1-5] Table 1-4. Nations Known or Suspected of Possessing Chemical Weapons
| KNOWN TO POSSESS | SUSPECTED OF POSSESSING |
|---|---|
| UNITED STATES OF AMERICA | PEOPLE'S REPUBLIC OF CHINA |
| RUSSIA | NORTH KOREA |
| FRANCE | EGYPT |
| LIBYA | ISRAEL |
| IRAQ[*] | ETHIOPIA |
| IRAN | TAIWAN |
| SYRIA | BURMA |
| [*] FOLLOWING THE PERSIAN GULF WAR (1990-91), THE UNITED NATIONS (UN) BEGAN DESTROYING CW MUNITIONS DISCOVERED DURING INSPECTION VISITS TO IRAQ BY UN ARMS CONTROL INSPECTORS. INCLUDED AMONG THE CW MUNITIONS DISCOVERED WERE SOME 2,000 AERIAL BOMBS AND 6,200 ARTILLERY SHELLS FILLED WITH MUSTARD AND SEVERAL THOUSAND 122 MILLIMETERS (mm) ROCKET WARHEADS FILLED WITH NERVE AGENT (GB). IRAQ ALSO DECLARED SURFACE TO AIR MISSILE (SCUD) WARHEADS FILLED WITH NERVE AGENT (GB AND GF). TABLE 1-5 PROVIDES A LIST OF KNOWN CW AGENTS. | |
Table 1-5. Chemical Warfare Agents
| NERVE | VESICANT | INCAPACITATING | CHOKING | BLOOD |
|---|---|---|---|---|
| TABUN (GA) | SULFUR MUSTARD (HD) | CNS DEPRESSANT (BZ) | PHOSGENE (CG) | HYDROGEN CYANIDE (AC) |
| GB | HL | CHLORINE (CL) | DIPHOSGENE (DP) | CYANOGEN CHLORIDE (CK) |
| GD | L | CHLOROPICRIN (PS) | ||
| GF | PHOSGENE OXIME (CX) | D-LYSERGIC ACID DIETHYLAMIDE (LSD) | ||
| VX |
d. Toxic Industrial Materials.
Toxic industrial materials can present a medical threat for deployed forces. Toxic industrial materials are comprised of toxic industrial biologicals (TIB), toxic industrial chemicals (TIC), and toxic industrial radiological (TIR) materials. These materials are found throughout the world and are used on a daily basis for commercial and private purposes. Large storage facilities, transportation tankers (over the road and railcars), as well as smaller containers of material, pose a danger to the health of personnel. Accidental spills or releases and terrorist actions can all lead to release of these materials into the environment causing potential casualty producing effects. Medical treatment facilities and nuclear power plants use radioactive materials that can pose a health hazard if accidentally released or used by hostile forces, terrorists, or others to contaminate an area. Biological materials used in medical research and pharmaceutical manufacturing may be used by hostile forces, terrorists, or others to produce casualties. Many TICs produce the same effects on personnel as CW agents. As a matter of fact, many TICs are of the same chemical structure as CW agents. However, there is quite a difference in their potency; in most TICs the potency is much lower. [1-6] For example, chlorine used to treat water supplies has also been used as a CW agent; organophosphate pesticides can cause the same effects as some nerve agents. Hostile forces, terrorists, or others may use RDDs to produce casualties as well. For detailed information on toxic industrial materials see FM 8-500.
The US forces may be attacked by or exposed to NBC, TIM, lasers, advanced electronics, high explosives, fuel-air, thermobaric, and conventional weapons; or a combination of these weapons/materiel. Mass casualty situations will be the rule and not the exception. Mass casualty situations can occur anyplace on the battlefield. Combined NBC and conventional weapons injuries may predominate. Command and control (C2) will be essential to prevent casualties and to provide effective HSS. However, C2 (to include HSS C2) elements may be primary targets. Effective HSS in an NBC environment can be accomplished, but only if necessary preparations to survive and to be mission capable are taken. Increased HSS C2 actions are needed to maintain HSS proximity to the supported force; to clear the battlefield; to move and resupply the HSS units, while managing multiple simultaneous mass casualty incidents; and to rapidly evacuate patients. Health service support C2 units must push HSS augmentation to mass casualty sites, clear the site, evacuate the patients to Medical Treatment Facilities (MTFs) that can provide essential care or out of the AO; decontaminate and extract medical forces from NBC contaminated areas and redistribute or redeploy the HSS forces. Within medical units, C2 will be challenged by the use of protective clothing and equipment, the need to move (either to the patients or out of the contaminated area), and obtaining additional support. Health service support advisers and staff officers must provide guidance to commanders on continued duty for personnel who have been exposed to NBC weapons/agents and TIM effects. Leaders must greatly increase coordinating, preplanning, using tactical standing operating procedures (TSOPs), and establishing multiple C2 mechanisms. See Appendix C for guidelines on operational planning for health service support in an NBC or TIM environment. See Appendix D for medical planning guide on NBC casualties. See Appendix E for a sample format of a "medical NBC staff officer appendix to annex Q."
a. Battle situational understanding is of great importance on the NBC battlefield. The number of casualties from each NBC attack will overwhelm any single medical unit or MTF causing the medical commander/leader to take action. To the extent possible, the commander/leader should be prepared for the requirement instead of reacting to it. To ensure responsive C2 the HSS plan must consider:
b. Clearing the battlefield will require preplanning and close coordination at all levels. Early resuscitation, stabilization, and prompt medical evacuation (MEDEVAC) are mandatory for survival of the sick and wounded.
c. For conventional operations C2 see FM 8-10. Field Manual 8-55 provides HSS planning for conventional operations.
d. Provisions for emergency medical care of civilians, consistent with the military situation. All non-DOD civilian care must be approved by the AO Commander in Chief/senior official and coordinated with the civil affairs unit and/or country team. For eligibility of care determinations guidance, see FM 8-10.
e. For additional information on planning operations in an NBC environment see FMs 8-10, 4-02.10, 4-02.4, 4-02.6, 4-02.283, 8-9, 8-10-6, 8-10-26, 8-284, and 8-285. Higher headquarters must distribute timely plans and directives to subordinate units to ensure that the subordinate unit's HSS plan supports their plan.
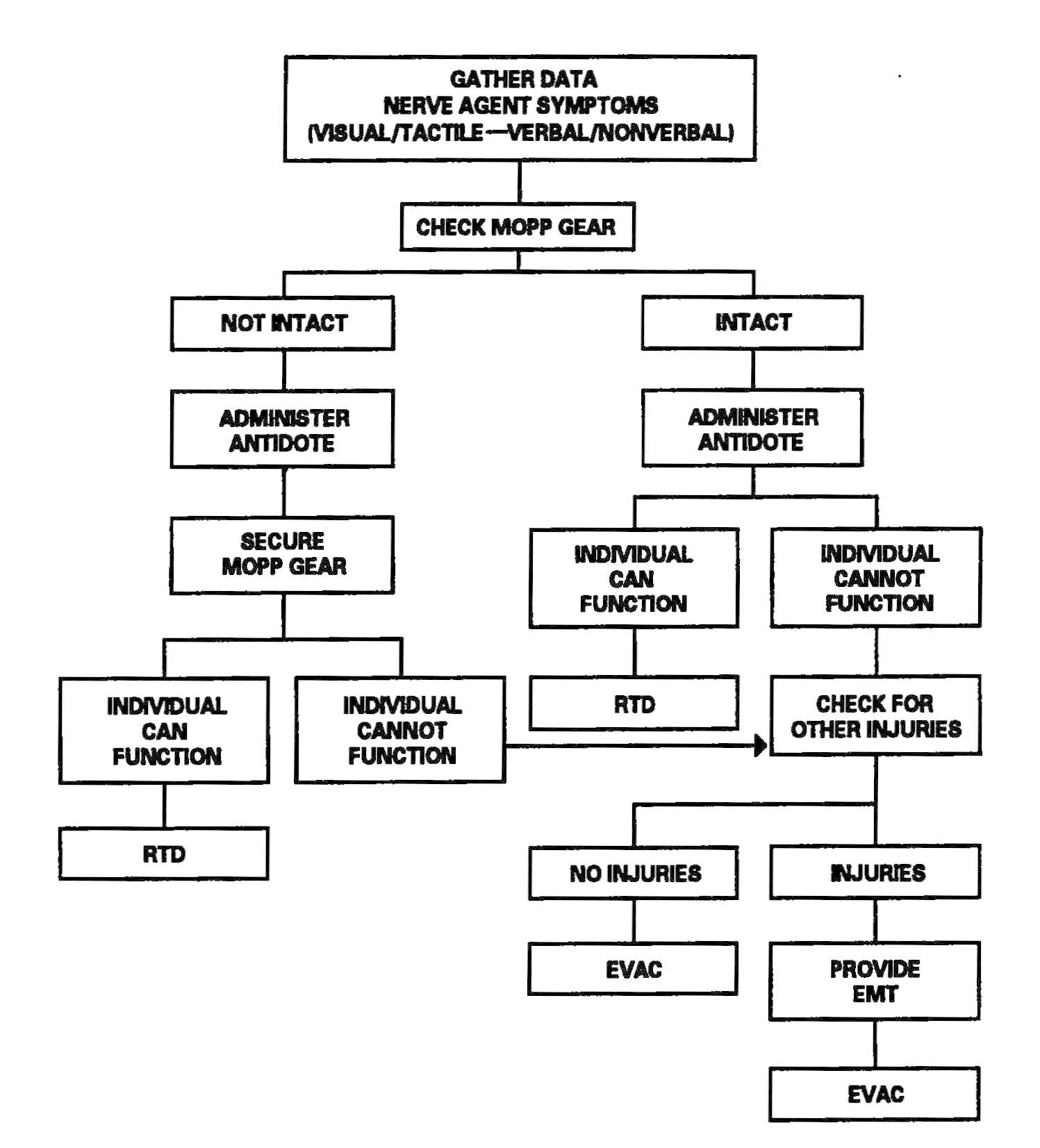
The HSS personnel make an appraisal of the supported mission to determine the expected patient load. Once the appraisal has been accomplished, HSS personnel prepare for the HSS mission by assigning personnel responsibilities. Using triage and EMT decision matrices for managing patients in a contaminated environment improves treatment proficiency. See Figure 2-1 for a sample decision matrix. Training HSS personnel in the use of simple decision matrices should enhance their effectiveness and contribute to a more efficient battlefield HSS process. Prior training for designated nonmedical personnel in patient decontamination procedures will enhance their effectiveness in the overall patient care mission. See Appendix D for planning factors on the estimation of NBC casualties.
Health service support units must plan, train, and routinely practice mass casualty management. The NBC attack or TIM event will likely be in conjunction with enemy conventional operations. But, the TIM event may be caused by terrorist or belligerent action. There will likely be increased conventional casualties in addition to the NBC/TIM related casualties. The supply and transportation units will be using the MSR in [2-3]support of the combat commander's requirements; thus, impacting on patient MEDEVAC and HSS unit resupply. Communications will be disrupted. Therefore, HSS C2 must plan and prepare for conducting operations with limited or no communications with other HSS organizations.

Figure 2-1. Sample triage and emergency medical treatment decision matrix.
Operations in a contaminated area require the HSS commander/leader to operate with contaminated or potentially contaminated assets. The following provides guidance in determining how to operate with contaminated facilities:
[2-4] a. Fulfill Health Service Support Principles. In making his decision to move or continue to operate with contaminated facilities, the commander/leader must apply the principles of conformity, proximity, flexibility, mobility, continuity, and control. The unit's operation must conform to the tactical commander's operation plan (OPLAN). Health service support must be provided to the tactical unit as far forward as possible; this ensures prompt, timely care. Additionally, the HSS commander/leader must be flexible; his support must be tailored to meet the supported commander's OPLAN requirements. Therefore, HSS assets must be as mobile as the unit they support. Finally, the HSS commander/leader must control his assets. Dispersion on the integrated battlefield may enhance unit survivability; but the HSS commander/leader may not be able to maintain control of his assets, they may become compromised.
b. Decision to Move. The HSS commander/leader (when deciding to move his unit to an uncontaminated area or in support of the tactical commander's plan) must base his decision to move on several factors.
(1) Protection available. What type of protection is available in the new area? Will he need to establish the units' collective protection shelter (CPS) systems, or are indigenous shelters available (for example, buildings, tunnels, caves)? Does the unit have sufficient individual protective equipment for unit personnel?
(2) Persistency. If his unit has been in a contaminated area, is the contamination persistent or nonpersistent? Is the area he will move to contaminated or clean? Persistency determines the MOPP level; the degree of threat; and performance decrement caused by the protective measures used. The level of contamination will determine whether employment of CPS is viable. The MTF may be able to continue to operate at the location by employing CPS. Personnel and patient decontamination must be accomplished before processing into the CPS.
(3) Patients. Before moving the entire facility, the HSS commander/leader must consider the number and types of patients at the MTF; his ability to redirect en route patients to the new MTF location; and his ability to evacuate the patients currently on hand. All patients should be stabilized before movement; but, MEDEVAC must be continued.
(4) Alternate facilities. Alternate facilities may be used (if the facility can be configured to ensure continuity of care or provide a protected area for patients) until the relocating activity is up and operating. This is a viable consideration when CPS is not available or the current location is contaminated with a persistent agent. Patient decontamination cannot be performed in an area heavily contaminated with a persistent agent.
(5) Medical evacuation. Consideration must always be given to the patient. Routes of MEDEVAC must be disseminated to supported and supporting units. The ability to evacuate patients during the move must continue. All MEDEVAC considerations must be addressed before any move.
(6) Mobility. An MTF that is not 100 percent mobile requires movement support. Thus, the commander/leader must coordinate movement support requirements with higher headquarters.
[2-5] (7) Mission. The primary consideration is the support mission of the MTF. The tactical commander requires continuous HSS for his personnel; when a move jeopardizes the quality of care, the move may be delayed.
(8) Sustainability. Hand-in-hand with the mission is sustainability (the ability of the unit to continue its support mission). If the current location of the MTF hinders the unit's ability to sustain its support mission, then the MTFs support to the unit is in question. Similarly, if moving the MTF will result in a disruption of support, then the move may not be viable.
(9) Decontamination. When a nonpersistent agent hazard exists and a CPS is not available, patients may be directed to another MTF until the hazard is gone; or the MTF can move to a contamination free area. Certain facilities may be decontaminated, patient protection procedures applied, and the operation continued. However, an MTF contaminated with a persistent agent requires time-consuming and resource-intensive decontamination operations; it may include replacement of contaminated shelters.
c. Management of Contaminated and "Clean" Facilities. Facilities contaminated with a persistent agent may be too resource intensive to decontaminate. Operating with a combination of contaminated assets and "clean" assets may be necessary. Mark contaminated assets with standard warning tags. Use these assets in contaminated environments and along contaminated routes. Keep clean assets in operation in clean areas. Of primary importance is proper marking and the avoidance of cross contamination.
d. Medical Supplies and Equipment for Patient Treatment. Are sufficient medical supplies and equipment available to perform the anticipated mission? Does the unit have special medical equipment sets available (chemical agent patient decontamination and chemical agent patient treatment medical equipment sets)?
a. Operating on a contaminated battlefield will stress leadership. Heat stress from being in higher levels of MOPP for long periods of time may lead to dehydration. The commander/leader must ensure that his personnel rest, drink, and eat sufficiently to allow them to continue with the mission. In the midst of activity, rest, hydration, and nutrition are often overlooked; however, a good leader will ensure that his personnel needs are met. See FM 21-10 for work/rest cycles and water drinking requirements. Individuals may suffer hyperventilation because of the enclosed feelings. Personnel remaining in MOPP Level 4 around the clock may suffer from increased sleep loss. Use of CPS can reduce this problem by allowing the personnel to rest out of their MOPP gear. Leaders must share leadership responsibilities and delegate responsibilities as much as possible so that each one gets sufficient rest to maintain unit effectiveness. Further, leaders should concentrate on supervision or unit mission, rather than on generation of new procedures during and after an attack. The NBC battlefield will, therefore, require more proactive and dedicated leaders who can balance the needs of their personnel and the mission. Further, leaders will be challenged by an additional logistics burden of providing nontraditional respiratory protection for personnel against TIMs. For detailed information on combat operational stress control (COSC) see FM 8-51 and FM 22-51.
DANGER
The standard NBC protective mask will not protect personnel from most TICs.
b. Leadership must plan for and establish procedures to maintain personnel performance during NBC operations. Personnel performance while wearing MOPP is degrading. At MOPP Level 3 or 4, all but the most basic patient care procedures may have to be suspended because—
Commanders and leaders must plan for and be prepared to support homeland security efforts; especially, for response to chemical, biological, radiological, nuclear, and high-yield explosive (CBRNE) events. Depending upon the location of the event, the response may be to a military installation in support of the weapons of mass destruction—installation support team (WMD-IST) or to an event site off a military installation. Response to a CBRNE event off a military installation will normally require a request for Department of Defense support to the event from the first responders to the event (usually from the incident commander or lead federal agency [Federal Bureau of Investigation or Federal Emergency Management Agency]). See Appendix C for planning considerations.
a. The use of NBC weapons is a condition of battle and HSS personnel must prepare to operate in these environments. Added is the dimension of TIM releases/incidents in the operational area. The importance of preventive medicine (PVNTMED) measures and first aid (self-aid, buddy aid, and combat lifesaver [CLS] support) are even more critical. Heat and stress injuries related to MOPP wear are issues for the HSS leadership as well as the force he is supporting. The stress load on personnel is increased by the concerns of being exposed to TIM releases. Considering that staffing of HSS units is based upon the minimum required to provide support on a conventional battlefield, they will be challenged to provide the same level of HSS in these environments.
b. The HSS leadership must quantify the HSS capability to their commanders. The medical staff must review OPLANS and make recommendations to reduce the number of patients. Medical NBC training programs must stress the essential imperative of immediate decontamination, the need to monitor your buddy for NBC and heat or combat/operational stress injury effects, and the proper use of NBC defense prophylaxis, pretreatments, insect repellents, barrier creams, and immunizations.
c. Maintaining close proximity to the supported force has been a major tenet of HSS doctrine and a critical factor in reducing the mortality rate. Maintaining this proximity and finding a place clean enough to provide necessary care requires intense coordination with the supported force. Alternate casualty collection points, decontamination sites, medical treatment sites, and MEDEVAC routes must be established, coordinated and communicated to the lowest level practical. Communication will be much more difficult, but must be maintained. Timely reports through the HSS technical channels will allow an optimal HSS response. Replacements for HSS front line losses must be rapidly filled after NBC weapons are employed.
d. Contamination (NBC and TIMs) can significantly hinder HSS operations. To maximize the unit's survivability and HSS capabilities and to avoid such contamination, leaders must use—
e. On the NBC battlefield, as on the conventional battlefield, HSS is focused on keeping soldiers in the battle. Effective and efficient PVNTMED measures, triage, emergency medical treatment (EMT), decontamination, advanced trauma management (ATM), and contamination control in the AO saves lives, assures judicious MEDEVAC, and maximizes the return to duty (RTD) rate.
a. Level I (unit-level) HSS may consist of a combat medic section, a MEDEVAC section, and a treatment squad. The treatment squad operates the Level I MTF (battalion aid station [BAS]). Level I HSS is supported by first aid in the form of self-aid/buddy aid and the CLS. See FM 4-02.4 for detailed information on conventional Level I HSS.
b. When operating under an NBC threat or when NBC attack is imminent, the BAS must prepare for continuation of its mission. Should an attack occur or a downwind hazard exist, the BAS must seek out a contamination free area to establish a clean treatment area, or must establish collective protection to continue the mission. Some BASs have Chemically Biologically Protected Shelter (CBPS) Systems. When available, these systems serve as the primary shelter for the BAS; they are operated in the full chemical/biological (CB) mode when attack is imminent or has occurred. See Appendix F for information on establishing a BAS in a CBPS system. When operating in the CB mode only patients requiring life- or limb-saving procedures are allowed entry at the BAS. Patients that have minor injuries that can be managed in the contaminated EMT area of the patient decontamination site will receive treatment in this area. After treatment, these patients will have the integrity of their MOPP restored by taping the damaged area and returned to duty. Patients with injuries that require further treatment, but who can survive evacuation to the Level II MTF will have their MOPP spot decontaminated, their injuries managed, the integrity of their MOPP restored, and be directed to an evacuation point to await transport to the Level II MTF (example, an individual with a splinted broken arm). When patients or personnel are contaminated or are potentially contaminated, they must be decontaminated before admission into the clean treatment area (see FM 3-5 for personnel decontamination procedures and Appendix G for patient decontamination procedures).
a. In the brigade, Level II HSS consists of—
b. In the division, HSS is the same as for the brigade, except patients may be evacuated from the BSA DCS, but not evacuated from the BAS.
c. When operating under an NBC threat or when NBC attack is imminent, the DCS must prepare for continuation of its mission. Should an attack occur or a downwind hazard exist the DCS must seek out a contamination free area, or must establish collective protection to continue the mission. The DCS in some medical companies have four CBPS Systems; they are complexed to provide space for DCS operations. These systems are operated in the CB mode when attack is imminent or has occurred. See Appendix F for information on establishing a DCS in CBPS Systems. When operating in the CB mode only patients requiring life- or limb-saving procedures are allowed entry. Patients with minor injuries that can be managed in the contaminated EMT area of the patient decontamination site will receive treatment in this area. After treatment, these patients will have the integrity of their MOPP restored by taping the damaged area and returned to duty. Patients with injuries that require further treatment, but who can survive evacuation to the Level III MTF will have their MOPP spot decontaminated, their injuries managed, and be directed to an evacuation point to await transport to the Level III MTF (example, an individual with a splinted broken arm). When personnel and patients are contaminated or are potentially contaminated, they must be decontaminated before admission into the clean treatment area (see FM 3-5 for personnel decontamination procedures and Appendix G for patient decontamination procedures).
Forward surgical teams (FST) are either organic to divisional and nondivisional medical units or are forward deployed in support of divisional or nondivisional medical companies to provide a surgical capability. Field Manual 8-10-25 describes FST operations. However, when forward deployed and NBC contamination is imminent the FST must employ collective protection in order to continue their support mission. When operating in a contaminated area the FST CBPS Systems must be complexed with the DCS CBPS. The FST cannot operate in an NBC environment without the support of the DCS. They do not have the capability to decontaminate patients. All patients are decontaminated in the DCS patient decontamination area. They are then processed into the EMT section of the DCS; where they are triaged and routed to the FST for surgery, if required. See Appendix F for FST employment of collective protection procedures.
a. Given the disruption of transportation, communications, and operations during and following an NBC attack, it should be clear that preparation is the key to survival and effectively providing HSS. Preparing a simple and complete TSOP and HSS plan that really integrates NBC is the first step. Critical training for medical personnel before an NBC attack is how to—
b. Even minimal site preparation (nuclear hardening or CB protecting) may improve survival, greatly reduce contamination, and maintain the ability to continue to provide HSS. See the discussion below for more information on each environment. As with other military personnel, HSS personnel must keep their immunizations current; use available prophylaxis against suspect CB agents; use pretreatments for suspect chemical agents; use insect repellents, and have antidotes and essential medical supplies readily available for known or suspected NBC effects. The best defense for HSS personnel is to protect themselves, their patients, medical supplies, and equipment by applying contamination avoidance procedures. They must ensure that stored medical supplies and equipment are in protected areas or in their storage containers with covers in place. One method of having supplies and equipment protected is to keep them in their shipping containers until actually needed. When time permits and warnings are received that an NBC attack is imminent, or that a downwind hazard exists, HSS personnel should employ their CPS (see Appendix F) or seek protected areas (buildings, tents, or other ABOVE ground shelters for biological or chemical attack; culverts, ravines, basements, or other shielded areas for nuclear) for themselves and their patients.
c. Other tasks include:
While it is possible that the NBC attack will be discrete short events, the more likely scenario is the enemy will use NBC throughout the conflict. The warning and reporting system will provide as much notice as is possible. Using the information provided, HSS personnel will continue their mission by using the best available protected areas. If warned of a nuclear attack, they take up positions within the best available shelter; movement out of these positions will be directed by leadership when it is safe to do so.
All personnel must survey their equipment to determine the extent of damage and their capabilities to continue the mission. Initially, patients from nuclear detonations will be suffering thermal burns or blast injuries. Also, expect patients and HSS personnel to be disoriented. Nuclear blast and thermal injuries will immediately manifest, most radiation-induced injuries will not be observed for several hours to days. Chemical agent patients will manifest their injuries immediately upon exposure to the agent, except for blister agents. [3-5] Biological agent patients may not show any signs of illness for hours to days after exposure, except for trichothecene (T2) mycotoxins. All patients arriving at Levels I and II MTFs must be checked for NBC contamination. Patients are decontaminated before treatment (see Appendix G) to reduce the hazard to HSS personnel, unless life- or limb-threatening conditions exist. Patients requiring treatment before decontamination are treated in the EMT area of the patient decontamination station. Examples of patient conditions that may require treatment at the contaminated treatment station of the patient decontamination area—
a. Health service logistics (HSL) personnel must train and prepare to operate in all battlefield situations. Operating in an NBC environment requires the issue of chemical patient treatment medical equipment set and chemical patient decontamination medical equipment set. Expect disruption of MSR and communications systems and plan accordingly. See FM 4-02.1 and FM 8-10-9 for details on HSL operations.
b. The medical platoon (Level I) is authorized two chemical agent patient treatment medical equipment sets and one chemical agent patient decontamination medical equipment set. Each chemical agent patient treatment medical equipment set has enough supplies to treat 30 patients. Each chemical agent patient decontamination medical equipment set has enough consumable supplies to decontaminate 60 patients.
NOTE
The chlorine granules in the chemical agent patient decontamination set are used to prepare the hypochlorite solutions for use to decontaminate patients.
c. The brigade, divisional, and nondivisional medical companies are authorized five chemical agent patient treatment medical equipment sets and three chemical agent patient decontamination medical equipment sets. These medical equipment sets are for use at the DCS patient decontamination station.
During NBC actions, HSS personnel requirements increase; thus, HSS reinforcement or replacements are necessary. Plans for HSS in a NBC battlefield must include efforts to conserve available HSS personnel and ensure their best use. HSS personnel will be fully active in providing EMT or ATM care; they will provide more definitive treatment as time and resources permit. However, to provide care they must be [3-6] able to work in a shirt-sleeved environment, not in MOPP Levels 3 or 4. Nonmedical personnel conduct search and rescue operations for the injured or wounded; they provide immediate first aid and decontamination. See FM 3-5, for detailed information on personnel and equipment decontamination operations. See FMs 4-02.283, 8-284, and 8-285 for detailed information on treatment of NBC patients.
a. Select sites for the BAS and DCS that are located away from likely enemy target areas. Cover and concealment is extremely important; they increase protection for operating the MTF.
b. Operating a CBPS System in the CB mode at the BAS requires at least eight medical personnel. The senior NCO performs patient triage and limited EMT and minor injury care in the patient decontamination area. One trauma specialist supervises patient decontamination and manages patients during the decontamination process. Two trauma specialists work on the clean side of the hot line and manage the patients until they are placed in the clean treatment area or are sent into the CBPS for treatment. They also manage the patients that are awaiting MEDEVAC to the DCS. The physician, physician assistant, and two trauma specialists provide ATM in the clean treatment area or inside the CBPS. See Appendix F for CPS entry/exit procedures.
c. When the BAS or DCS are receiving NBC contaminated patients, they require at least eight nonmedical personnel from supported units to perform patient decontamination procedures. These facilities are only staffed to provide patient care under conventional operational conditions. Without the augmentation support, they can either provide patient decontamination or patient care, but not both.
d. A patient decontamination station is established to handle contaminated patients (see Appendix G). The station is separated from the clean treatment area by a "hot line" and is located downwind of the clean treatment area or CPS. Personnel on both sides of the "hot line" assume a MOPP level commensurate with the threat agent employed (normally MOPP Level 4). The patient decontamination station should be established in a contamination-free area of the battlefield. However, it may be necessary to establish a patient decontamination station that is collocated with an MTF that is employing a CBPS, in a chemical vapor hazard area in order to decontaminate patients and clear the battlefield before moving the MTF to a clean area. When CPS systems are not available, the clean treatment area is located upwind 30 to 50 meters of the contaminated work area. When personnel in the clean working area are away from the hot line, they may reduce their MOPP level. Chemical monitoring equipment must be used on the clean side of the hot line to detect vapor hazards due to slight shifts in wind currents; if vapors invade the clean work area, HSS personnel must re-mask to prevent low-level CW agent exposure and minimize clinical effects (such as miosis).
Civilian casualties may become a problem in populated or built-up areas, as they are unlikely to have protective equipment and training. The BAS and DCS may be required to provide assistance when civilian medical resources cannot handle the workload. However, aid to civilians will not be undertaken without command approval, or at the expense of health services provided to US personnel.
a. The HSS mission must continue in a nuclear environment; protected shelters are essential to continue the support role. Well-constructed shelters with overhead cover and expedient shelters (reinforced concrete structures, basements, railroad tunnels, or trenches) provide good protection from nuclear attacks (see Appendix H). Armored vehicles provide some protection against both the blast and radiation effects of nuclear weapons. Patients generated in a nuclear attack will likely suffer multiple injuries (combination of blast, thermal, and radiation injuries) that will complicate medical care. Nuclear radiation patients fall into three categories:
b. Medical units operating in a radiation fallout environment will face three problems:
c. Decontamination of most radiological contaminated patients and equipment can be accomplished with soap and water. Soap and water will not neutralize radioactive material. However, it will remove the material from the skin, hair or material surface. See Appendix G for specific patient decontamination procedures. The waste can become a concentrated point of radiation and must be managed and monitored.
d. Commanders and leaders must consider the radiation exposure levels for themselves, their staffs, and patients when operating in or determining if the unit will enter a radiologically contaminated [3-8] area. The commander and leader must establish an operational exposure guide for their unit and personnel. The operational exposure guide (OEG) is established for either battlefield exposures as shown in Table 3-1 or for exposures in stability operations and support operations as shown in Table 3-2. The tables present radiation exposure status (RES) categories; however, they can be used to establish OEGs based on the same exposure criteria.
Table 3-1. Radiation Exposure Status Categories for Tactical Operations
| RES-O | THE UNIT HAS HAD NO RADIATION EXPOSURE. |
| RES-1 | THE UNIT HAS BEEN EXPOSED TO GREATER THAN 0 cGy BUT LESS THAN OR EQUAL TO 75 cGy. |
| RES-2 | THE UNIT HAS BEEN EXPOSED TO GREATER THAN 75 cGy BUT LESS THAN OR EQUAL TO 125 cGy. |
| RES-3 | THE UNIT HAS BEEN EXPOSED TO GREATER THAN 125 cGy. |
Table 3-2. Radiation Exposure Status Categories During Stability Operations and Support Operations
| RES-O | <0.05 cGy | ||
| RES-1A | 0.05 TO 0.5 cGy | ||
| RES-1B | 0.5 TO 5 cGy | ||
| RES-1C | 5 TO 10 cGy | ||
| RES-1D | 10 TO 25 cGy | ||
| RES-1E | 25 TO 75 cGy |
Medical triage is the classification of patients according to the type and seriousness of illness or injury; this achieves the most orderly, timely, and efficient use of HSS resources. However, the triage process and classification of nuclear patients differs from conventional injuries. See FM 4-02.283 for nuclear patient triage and treatment procedures.
a. A biological attack (such as the enemy use of bomblets, rockets, spray or aerosol dispersal, release of arthropod vectors, and terrorist or insurgent contamination of food and water) may be difficult to recognize. Frequently, it does not have an immediate effect on exposed personnel. All HSS personnel must monitor for BW indicators such as—
b. Passive defensive measures (such as immunizations, good personal hygiene, physical conditioning, using arthropod repellents, wearing protective mask, and practicing good sanitation) will mitigate the effects of many biological agent intrusions.
c. The HSS commanders and leaders must enforce contamination control to prevent illness or injury to HSS personnel and to preserve the facility. Incoming vehicles, personnel, and patients must be surveyed for contamination. Ventilation systems in MTFs (without CPS) must be turned off if BW exposure is imminent.
d. Decontamination of most BW contaminated patients and equipment can be accomplished with soap and water. Soap and water will not kill all biological agents; however, it will remove the agent from the skin or equipment surface. See Appendix G for specific patient decontamination procedures.
e. Treatment of BW agent patients may require observing and evaluating the individual to determine necessary medications, isolation, or management. See FM 8-284 for specific treatment procedures for BW agent patients.
f. Medical surveillance is essential. Most BW agent patients initially present common symptoms such as low-grade fever, chills, headache, malaise, and coughs. More patients than normal may be the first indication of biological attack. Daily medical treatment summaries, especially DNBI, need to be prepared and analyzed. Trends of increased numbers of patients presenting with unusual or the same symptoms are valuable indicators of enemy employment of BW agents. Daily analysis of medical summaries can provide early warnings of BW agent use, thus enabling commanders to initiate preventive measures earlier and reduce the total numbers of troops lost due to the illness. See FM 4-02.17 for information of medical surveillance procedures. See FM 8-284 for preventive, protective, and treatment procedures.
a. Consider that all patients generated in a CW agent environment are contaminated. The vapor hazards associated with contaminated patients may require HSS personnel to remain at MOPP Level 4 for long periods. The MTF must be set up in clean areas or employ CPS. If there is liquid agent contamination, or a continued vapor hazard, the MTF should be moved and be decontaminated, mission permitting.
b. Initial triage, EMT, and decontamination are accomplished on the "dirty" side of the hot line. Life-sustaining care is rendered, as required, without regard to contamination. Normally, the senior health care sergeant performs initial triage and EMT at the BAS. Secondary triage, ATM, and patient disposition are accomplished on the clean side of the hot line. When treatment must be provided in a contaminated environment outside the CPS, the level of care may be greatly reduced because medical personnel and patients are in MOPP Level 3 or 4. However, lifesaving procedures must be accomplished. See FM 8-285 for specific treatment of CW agent patients.
c. Decontamination of most chemically contaminated patients and equipment requires the use of materials that will remove and neutralize the agent. See FM 3-5 for equipment decontamination procedures and Appendix G for specific patient decontamination procedures.
Enemy employment of NBC weapons or TIMs in the extremes of climate or terrain warrants additional consideration. Included are the peculiarities of urban terrain, mountains, snow and extreme cold, jungle, and desert operations in an NBC environment with the resultant NBC-related effects upon medical treatment and MEDEVAC. For a more detailed discussion on NBC aspects of urban terrain, mountain, snow and extreme cold, jungle, and desert operations, see FMs 3-06.11, 31-71, 90-3, 90-5, and 90-10.
a. In mountain operations, passes and gorges may tend to channel the nuclear blast and the movement of chemical and biological agents. Ridges and steep slopes may offer some shielding from thermal radiation effects. Close terrain may limit concentrations of troops and fewer targets may exist; therefore, a lower patient load may be anticipated. However, the terrain will complicate patient evacuation and may require patients to be decontaminated, treated, and held for longer periods than would be required for other operational areas.
b. The effects of extreme cold weather combined with NBC-produced injuries have not been extensively studied. However, with traumatic injuries, cold hastens the progress of shock, providing a less favorable prognosis. Thermal effects will tend to be reinforced by reflection of thermal radiation from snow and ice-covered areas. Care must be exercised when moving chemically contaminated patients into a warm shelter. A CW agent on the patient's clothing may not be apparent. As the clothing warms to room temperature, the CW agent will vaporize (off-gas), contaminating the shelter and exposing occupants to potentially hazardous levels of the agent. A three-tent system is suggested for processing patients in extreme cold operations. The first tent (unheated) is used to strip off potentially contaminated clothing. The second (heated) is used to perform decontamination, perform EMT and detect off gassing. The third (heated) is used to provide the follow on care and patient holding.
c. In rain forests and other jungle environments, the overhead canopy will, to some extent, shield personnel from thermal radiation. However, the canopy may ignite and create forest fires and result in burn injuries. By reducing sunlight, the canopy may increase the persistency effect of CW agents near ground level. The canopy also provides a favorable environment for BW agent dispersion and survival.
d. In desert operations, troops may be widely dispersed, presenting less profitable targets. However, the lack of cover and concealment exposes troops to increased hazards. Smooth sand is a good reflector of nuclear thermal and blast effects; generating increased numbers of injuries. High temperatures will increase the discomfort and debilitating effects on personnel wearing MOPP, especially heat injuries.
a. An NBC environment forces the unit leadership to consider to what extent he will commit MEDEVAC assets to the contaminated area. If the battalion or task force is operating in a contaminated [3-11] area, most or all of the organic medical platoon MEDEVAC assets will operate there. However, efforts should be made to keep some ambulances free of contamination. For conventional MEDEVAC operations see FM 8-10-6 and FM 8-10-26.
b. We have three basic modes of evacuating patients (personnel [litter bearers], ground vehicles, and aircraft). Using litter bearers to carry the patients involves a great deal of stress. Cumbersome MOPP gear, added to climate, increased workload, and the fatigue of battle, will greatly reduce personnel effectiveness. If personnel must enter a radiologically contaminated area, an OEG must be established (see Table 3-1). Radiation exposure records are maintained by the NBC NCO and made available to the commander, staff, and medical leader. The exposure is entered into the individual's medical record. Based on the OEG, the commander and leaders will decide which MEDEVAC assets will be sent into the contaminated area. Again, every effort is made to limit the number of MEDEVAC assets that are contaminated. Medical evacuation considerations should include the following:
(1) A number of ambulances will become contaminated in the course of battle. Optimize the use of resources; use those already contaminated (medical or nonmedical) before employing uncontaminated resources.
(2) Once a vehicle or aircraft has entered a contaminated area, it is highly unlikely that it can be spared long enough to undergo thorough decontamination. However, operational decontamination should be performed to the greatest extent possible. This will depend upon the contaminant, the tempo of the battle, and the resources available to the MEDEVAC unit. Normally, contaminated vehicles (air and ground) will be confined to dirty environments. See FM 3-5 for details on decontamination procedures.
(3) Use ground ambulances instead of air ambulances in contaminated areas; they are more plentiful, are easier to decontaminate, and are easier to replace. However, this does not preclude the use of aircraft. If an air ambulance is deployed into a contaminated area, use it for repeated MEDEVAC missions rather than sending other clean aircraft into the area.
(4) The relative positions of the contaminated area, forward line of own troops (FLOT), and threat air defense systems will determine where helicopters may be used in the MEDEVAC process. One or more helicopters may be restricted to contaminated areas; use ground vehicles to cross the line separating clean and contaminated areas. The ground ambulance proceeds to an MTF with a patient decontamination station (PDS); the patient is decontaminated and treated. If further MEDEVAC is required, a clean ground or air ambulance is used. The routes used by ground vehicles to cross between contaminated and clean areas are considered dirty routes and should not be crossed by clean vehicles, if mission permits. Consider the effects of wind and time upon the contaminants; some agents will remain for extended periods of time.
(5) Keep the helicopter rotor wash in mind when evacuating patients, especially in a contaminated environment. The intense rotor wash will disturb the contaminants and further aggravate the condition. The aircraft must be allowed to land and reduce to flat pitch before patients are brought near. This will reduce the effects of the rotor wash. Additionally, a helicopter must not land too close to a decontamination station (especially upwind) because any trace of contaminants in the rotor wash will compromise the decontamination procedure.
c. Immediate decontamination of rotor wing aircraft and ground vehicles is accomplished to minimize crew exposure. Units include decontamination procedures in their standing operating procedures (SOP). A sample aircraft decontamination station that may be tailored to a unit's needs is provided in FM 3-5.
d. Evacuation of patients must continue, even in an NBC environment. The HSS leader must recognize the constraints NBC places on operations; then plan and train to overcome these deficiencies.
e. To minimize the spread of contamination inside the MEDEVAC aircraft, plastic sheeting should be placed under the litter to catch any contaminant that drips off the patient or litter. The plastic sheeting can be removed with the patient, removing any contamination with it. When plastic sheeting is not available, placing a blanket under the litter will reduce the amount of agent that makes contact with the inside of the aircraft.
NOTE
The key to mission success is detailed preplanning. A HSS plan must be prepared for each support mission. Ensure that the HSS plan is in concert with the tactical plan. Use the plan as a starting point and improve on it while providing HSS.
f. Medical evacuation by United States Air Force (USAF) aircraft will be severely limited until runway repairs and decontamination has occurred. Aerial flights from contaminated areas into uncontaminated airspace and destinations may be impossible for extended periods of time; some nations will not allow patients from contaminated areas to travel through or over their country. Therefore, patient holding on-site (or in theater) for an extended period of time must be anticipated.
g. Patient protection during evacuation must be maintained. Patients that have been decontaminated at the PDS at an MTF will have had their MOPP ensemble removed. The forward deployed MTFs will not have replacement MOPP ensembles for the patients. These patients must be placed in a patient protective wrap (PPW) before they are removed from the clean treatment area for evacuation (see the PPW instruction sheet/PPW label for use of the PPW). The PPW provides the same level of protection as the MOPP ensemble. The patient does not have to wear a protective mask when inside the PPW. The patient is placed inside the PPW that is on a litter. The PPW may also have a battery-operated blower that can provide a reduction of the body heat load and reduce the carbon dioxide level within the PPW. The PPW will provide protection for the patient for up to 6 hours and is a one-time use item. The blower is reusable, remove it and the attachment devices from the used PPW and return it to the patient movement items inventory. See FM 4-02.1 for a discussion on patient movement items.
WARNING
DO NOT place contaminated patients in the PPW. It is for use with uncontaminated/decontaminated patients only.
a. Many factors must be considered when planning for Levels III and IV hospital support on the integrated battlefield. The hospital staff must be able to defend against threats by individuals or small groups (two or three) of infiltrators and survive NBC strikes or TIM incidents while continuing their mission. This threat may include the introduction of NBC or TIM in the hospital area, the water or food supplies; and the destruction of equipment and/or supplies. On the larger scale of surviving NBC strikes and continuing to support the mission, operating in a contaminated environment will present many problems for hospital personnel. The use of NBC weapons or TIM release can compromise both the quality and quantity of health care delivered by medical personnel due to the contamination at the MTF; constrain mobility and evacuation; and contaminate the logistical supply base. While providing hospital support, consider the following assumptions:
(1) Their location, close to other support assets, makes them vulnerable to NBC strikes and release/dispersion of TIMs.
NOTE
When using existing civilian hospitals, the materials for an RDD may be at these hospitals. Exploding the material in place is very practical for a small team of terrorists.
(2) Large numbers of casualties are produced in a short period of time. Many of these casualties may have injuries that are unfamiliar to hospital personnel. These injuries may include—
(3) In addition to the wounding effects of NBC weapons on troops, their use will have other effects upon the patient care delivery system.
(4) Mission-oriented protective posture reduces the efficiency of all personnel.
[4-3] (5) Without CPS systems, hospitals may operate for a limited time in a nonpersistent agent environment, but are incapable of operating in a persistent agent environment.
b. There are currently two force modernization initiative hospital systems in the force structure. The Medical Force 2000 (MF2K) system consists of the CSH, the field hospital (FH), and the general hospital (GH). The Medical Reengineering Initiative (MRI) consists of only one hospital system—the CSH. The MF2K CSH is a corps asset, whereas, the FH and GH are the echelon above corps hospital systems. The MRI CSH will be located in the corps and at echelons above corps. The MRI CSH will replace the FH and GH at echelons above corps. See FM 4-02.10, FM 8-10-14, and FM 8-10-15 for detailed information on these hospital systems.
a. Protection of hospital assets requires intensive use of intelligence information and careful planning. The limited mobility of hospitals makes their site selection vital to minimize collateral damage from attacks on other units.
b. Many defensive measures will either impede or preclude performance of the hospital mission. Successful hospital defense against an NBC threat is dependent upon accurate, timely receipt of information via the nuclear, biological, and chemical warning and reporting system (NBCWRS). This information will allow the hospital to operate longer without the limitations and problems associated with the use of the CPS and personnel assuming MOPP Levels 3 and 4. The detailed information (provided in the NBC 5 and 6 reports respectively) on the areas affected and the types of agents used allows the hospital staff to—
(1) Protective procedures.
(a) Because most hospital sections operate in sheltered areas (tentage or hard-walled shelter), some protection is provided against vapor, liquid, and particulate (fallout) hazards. Sealing all openings can increase the temporary protection from such hazards; all entries and exits must be curtailed while operating in this mode. Liquid agents will eventually seep through the tent fabric and create a vapor hazard inside the shelter. Locating equipment, such as trucks, under trees or other cover provides similar effects. Setting up hospitals in existing structures (concrete or steel buildings) provides greater protection from hazards and eliminates many decontamination problems. However, without means to seal openings, chemical agent vapors can enter the structure. The addition of CB filtration systems with air locks, that provide overpressure, can provide maximum protection for occupants. Entry and exit procedures must be established to prevent contamination being introduced by personnel and patients entering. See Appendix F for entry/exit procedures when CB filters and air locks are in use.
(b) Concealment and good operations security (OPSEC) will help prevent identification of a unit.
(c) Dispersion is a defensive measure employed by tactical commanders; however, hospital operations limit the value of this technique. One technique that may be used is locating sections of the hospital, such as the motor pool, personnel billets, laundry, and logistical storage, a greater distance from the hospital complex than normal. This will increase dispersion without severely compromising the hospital mission.
[4-5] (d) The MOPP ensemble does not protect against all radiation effects of nuclear weapons. However, it provides some protection against alpha and beta radiation burns. By covering all body surfaces, especially hairy areas, MOPP greatly expedites the decontamination process.
(2) Nuclear.
(a) Most protective measures for hospitals against nuclear attack require engineer and/or intensive logistic support. This support includes placing sandbag walls around tents; digging trenches for patient occupation; or constructing earthen berms (see Appendix H). Occupying existing structures, depending upon their strength and potential flammability, may be the best protection against the effects of a nuclear strike. The remainder of this section presents factors to consider when selecting the protective posture for the hospital against a nuclear attack. Leaving equipment packed and loaded until actually needed for operations will help protect materiel in an NBC environment. In any event the unit must have established an OEG, implemented radiation monitoring, and have contingency plans if these radiation levels are approached or exceeded.
(b) Personnel and patient protection requirements will depend upon the threat (blast, thermal, immediate radiation, or fallout effects). The MOPP ensemble will not protect against internal radiation, but will provide some protection from external radiological contamination.
(3) Biological. The most likely use of a biological agent (such as anthrax) is releasing the agent as an aerosol. While such agents may produce large numbers of casualties, initially patients may be seen at the MTF in small numbers, but the number of patients will rapidly increase within a few hours to days. When a trend is identified, the enemy use of a biological agent is suspected. General protective measures are the same as for any infectious disease; specific protective measures are used once the vector or method of transmission has been identified. Designating a single hospital to care for these patients (from a patient care or disease transmission standpoint) may not be necessary. However, if the agent is communicable, consolidating them all at one facility maximizes the use of limited assets and aids in limiting the spread of the disease. Protective measures against biological attack are the same as those for chemical agents when bombs, sprays, or gases are used; see (4) below. The difficulty in rapidly identifying biological agents may force the use of protective measures for longer periods of time. Faced with this situation, a careful evaluation of the mask-only posture is necessary before implementing any level of MOPP. See FM 8-284 for additional information on prevention, protection, and treatment of biological casualties.
(4) Chemical.
(a) Individual protection. When CPS systems are not available, using the correct MOPP level is essential in hospital mission performance. The level of MOPP assumed depends upon the level of threat. An alternative approach for the hospital commander is the use of the mask-only posture. This posture is acceptable when the hazard is from vapor only (except mustard). See FM 3-4 for a description of each MOPP level and mask-only procedures.
NOTE
Patients with injuries that prevent their assuming a protective posture must be placed in a PPW or immediately evacuated to a clean MTF.
(b) Environmental protection. As noted previously, hospital complexes without CPS offer some protection against liquid or fallout contamination, but little protection against vapor hazards.
(c) Patient protection.
CAUTION
Remember, personnel must assume MOPP Level 4 before beginning any decontamination process or risk becoming a casualty themselves.
(d) Materiel protection. Protection of materiel, especially expendable supplies, requires covers and barriers. All materiel not required for immediate use is kept in shipping containers, medical chests, or under cover (tentage, plastic sheeting, and tarpaulin) for protection against particulate or liquid hazard. Protection against vapor hazard may require multiple barriers through which the vapor must penetrate. For example, intravenous solutions are in their individual plastic bags, in the cardboard shipping box, on a covered pallet, in a hard-walled shelter; such as a military-owned demountable container (MILVAN). This presents four barriers against the vapor hazard. These principles should be used to the maximum extent practical.
a. Decontamination of nuclear-contaminated personnel, equipment, and the operational site is as follows:
(1) Monitoring equipment is used to detect contamination; the contamination is then removed by brushing or scraping with brooms, brushes, or tree branches. Flushing hard surface contaminated areas with water is also effective in removing nuclear contamination. However, there remains a problem of containing and removing the contaminated water. The best method of containment is to trench the area into a sump for collection of the contamination. This will reduce the area of contamination; however, the level of concentrated radiation may be such that there is an increased hazard to personnel. The collection area must be clearly marked using the standard nuclear hazard signs.
[4-9] (2) Nuclear contamination of the site may require relocating the hospital. Scraping 1 or 2 inches of topsoil from the area, or covering the area with 1 or 2 inches of uncontaminated dirt will not be practical. A need to relocate the hospital will depend upon the degree of contamination; the amount of decontamination possible and the projected stay before a normal move in support of operations. If the hospital is immersed in a high level of radioactivity, the best option may be to abandon it for 48 to 72 hours. After this period the area should be checked and if the radioactivity has decayed sufficiently the hospital may be reoccupied and continue operations or moved to a clean area. The command OEG must be followed if reoccupying or moving the facility.
b. Suspect biological agents should be removed from equipment as quickly as possible, In the absence of agent-specific guidance, clean exposed surfaces using a 5 percent hypochlorite solution or copious quantities of soap and water (preferably hot). Liberally apply the hot, soapy water and scrub all surfaces with a brush. Then rinse the surfaces with hot water. As previously discussed, the soapy water used is contaminated and must be controlled and removed to a safe area. Supertropical bleach (STB) and decontaminating solution Number 2 (DS2, US Army) are effective against most known biological agents because of their caustic nature. If anthrax (or other spore formers) is suspected, repeat the entire decontamination process again to remove the spores. Other standard biological decontamination agents are described in FM 3-5.
CAUTION
1. Keep liquid decontaminants out of equipment with electronic
or electrical circuits. Unplug electrical devices before attempting
to decontaminate them; prevent electric shock. Some electronic
devices maintain an electric charge, even after being unplugged,
use extreme care to prevent shock.
2. Soap and water only mechanically remove BW agents. The
soap and water solution must be contained to prevent spreading
the agent to other personnel, thus causing more casualties.
c. Decontamination of chemical contamination is as follows:
(1) Personnel use their soldier skills and their M295 Individual Equipment Decontamination Kit to decontaminate their personal equipment. The M13, decontamination apparatus, portable, is used to decontaminate vehicles, trailers, and International Organization for Standardization (ISO) shelters. This apparatus uses DS2 (a highly caustic, flammable solution that cannot be used to decontaminate tentage). The DS2 must be washed off after sufficient time has passed for decontamination (see FM 3-5 for details). Water used for NBC decontamination purposes becomes contaminated; therefore, it must be contained in sumps. Dig shallow trenches to channel the water into sumps. This will be difficult in hospital areas because relatively flat sites are needed for hospital complexing, but must be accomplished to reduce the contamination levels in the hospital area.
[4-10] (2) When hospital tentage becomes contaminated, decontamination operations must be considered immediately. Spot decontamination may be effective for small areas; however, gross contamination of TEMPER and GP tentage is best decontaminated by aging. Without CPS and with persistent agent contamination that absorbs into the tentage and presents a continuing vapor hazard, the hospital stops receiving patients and evacuates all patients as quickly as possible. When large portions of the hospital are contaminated, personnel decontaminate all equipment possible and relocate to a new site, leaving the contaminated equipment to age or to be decontaminated by a specialized unit. When small portions of the hospital are contaminated, the contaminated portions are removed to another location for decontamination; hospital operations are continued, but at a lower operational level. For detailed equipment decontamination procedures, see FM 3-5.
NOTE
Liquid decontamination material must not be used on electrical or electronic components of equipment. Liquid decontaminants can damage the equipment; thus making it inoperable and not available for patient care or transport. The use of liquids to decontaminate electronic equipment could also potentially result in injury or electrocution of personnel.
(3) Each US Army hospital is issued five chemical agent patient treatment MES and three chemical agent patient decontamination MES, Chemical Agents Patient Decontamination, for use in decontaminating patients. Each hospital must decontaminate and treat its own personnel who become casualties; chemical casualties from units in its general area; or contaminated patients received from lower level MTFs. See Appendix G for patient decontamination procedures and for establishment of a patient decontamination and treatment station.
a. Providing emergency services will be complicated by several factors:
b. Contaminated patients must be triaged in the decontamination area that is established at the hospital. Contaminated patients WILL NOT be brought into the clean EMT area until decontaminated. All patients are screened for contamination. Based on the findings, the patient is routed to the contaminated triage station, or to the clean triage station. Contaminated patients are triaged, then routed to the decontamination area, or to the contaminated treatment area. Patient admission to the clean treatment area may be delayed; however, life- or limb-saving care is provided in the contaminated treatment area before decontamination.
The provision of general medical services in the hospital will be continued with minimal interruptions in the NBC environment. The noninvasive nature of these services allows their continuation at most MOPP levels. However, some general medical services will be constrained by MOPP Levels 3 and 4 and the mask-only posture. These constraints may include, but not be limited to—
a. Surgical services will be severely limited in the NBC environment. At any level above MOPP Level 0, without a CPS system surgical services are halted except for life- or limb-saving expedient procedures. Surgery cannot be safely performed outside a CPS due to a variety of factors including—
b. Due to the relatively high number of trauma cases, hospital services may be severely constrained by NBC contamination. The hospital location and the possible need for relocation are two major planning considerations for the command staff.
c. Patient accounting and medical regulating are critical factors in the transfer of patients from a hospital without a CPS that must move out of an NBC environment. Hospitals without CPS stop receiving patients when a persistent hazard is identified; patients on hand are protected and transferred to a clean MTF.
Providing nursing care in a hospital without CPS is influenced by the amount of protective gear worn by the nursing staff and the patients. The patients may be in their MOPP gear, in a PPW, or wearing only their protective mask; any of which will interfere with care. The nursing staff will wear the same level of protection as the patients.
a. Direct assessment of a patient's vital signs is extremely limited at MOPP Levels 3 or 4; however, a carotid artery pulse can be taken by palpating the neck area. The patient's respiratory rate and level of consciousness may be assessed visually. Palpitation of the blood pressure through a PPW may be possible if it is relatively strong, or at least in the normal range. The patient's temperature cannot be monitored; this is an area of concern due to the possibility of heat stress.
b. Only gross neurological signs can be assessed through the PPW or when the patient is in MOPP Levels 3 or 4. However, even this assessment is complicated by the presence of miosis and by the health care provider's mask. Urinary output and cardiac monitoring is continued uninterrupted for patients wearing a mask only and for patients in the PPW.
c. Oral hygiene and bathing are postponed until a safe environment is available (MOPP Level 2 or less). All toileting will occur within the hospital complex using ISO contained latrines, chemical toilets, bedpans, urinals, buckets, or containers with plastic liners. Waste from improvised containers must be placed in containers with covers or in plastic bags and sealed to control odors and prevent spread of infectious material within the facility.
[4-13] d. At MOPP Levels 3 and 4, feeding must be postponed. A nutritional assessment is needed to determine how long each patient can tolerate a fasting state when MOPP Level 3 or Level 4 remains in effect for over 24 hours.
e. Intravenous (IV) medications are mixed in a clean area and then transported in a protective wrap (multilayers of plastic, medical chest, or layered cardboard) to the user. However, IV solutions, blood, and injections can be given to patients on an unprotected ward. Normally, oral medications are only given at MOPP Level 2 or lower.
f. Treatment procedures that have the potential of contaminating the patient's pulmonary or circulatory systems are conducted only at MOPP Level 2 or below. However, EMT procedures may have to be performed in the contaminated treatment area, or the patient decontamination area.
g. Continuous oxygen therapy requires a collective protection environment or a CB filter supported respirator.
h. Delivery of nursing care at MOPP Level 3 or Level 4 is limited due to the sensory restrictions of MOPP gear. Time is taken to reassure the patients on a personal basis, as much as possible, and by routinely monitoring the ward environment. Communications are difficult and identities are masked. Maintain the identity of personnel by using handwritten name tags for staff and patients (including patients in PPW).
i. As with all procedures, the time required for record-keeping rises markedly at MOPP Level 3 or Level 4. Contaminated paperwork cannot be evacuated with the patient. Transcribe essential information onto uncontaminated documents for evacuation with the patient. A record of patient exposure time to a contaminated area is prepared to assess the cumulative risk to the patient.
For conventional operations of hospitals in a field environment see FM 4-02.10, FM 8-10-14, and FM 8-10-15.
On the integrated battlefield, PVNTMED services will be in greater demand than at any other time, especially under BW conditions. Preventive medicine personnel will be called upon to assist the commander in determining the health hazards associated with nuclear fallout; the safety of drinking water in an NBC environment; as well as determining when to use prophylaxis, pretreatments, immunizations, and other PVNTMED measures (PMM) associated with NBC warfare. Preventive medicine personnel must be aware of the medical threat in the AO. They must continually update their medical surveillance activities to identify disease trends (endemic and epidemic), potential disease vectors, and the susceptibility of troops to these diseases. Under NBC conditions, diseases may manifest that exist in the area, but were not being transmitted to personnel. However, due to the reduced health status of personnel from exposures to or from stress-related NBC conditions, the troops begin to suffer their effects. The appearance of diseases or arthropods not known to exist in the AO are indicators that BW agents have been used. For details on PVNTMED operations, see FM 4-02.17.
a. Determining Factors. Factors of prime importance in determining the nature and severity of the disease effects are—
Finally, the manner and situation in which nuclear weapons are used are of importance. A single weapon detonated in a socially stable area will have far less serious effects than a detonation in an area where combat has already disrupted the social stability. At Hiroshima and Nagasaki, Japan (excellent examples of the first type of situation), the survivors who could get away were able to obtain food, shelter, and care from surrounding intact areas. With prolonged combat operations, such intact areas would not be available, resulting in no food, shelter, or care for survivors. There will be a breakdown in social order and there will be a lack of effective medical support; including PVNTMED functions and facilities.
[5-2] b. Disease Incidence. Without PVNTMED capabilities, increased incidence and morbidity from diseases will follow. Some diseases will predominate in incidence, depending upon the geographical areas involved and the endemic diseases present.
(1) In urban areas in temperate climates, several diseases are epidemic threats. These epidemic threats may include—
(2) There are several reasons for the increased risk of disease including, but not limited to—
The PVNTMED sections of the brigade, divisional, and nondivisional medical companies perform analysis on water sources and supplies to determine the presence or absence of NBC/TIM contamination; see Appendix I for additional information. Based upon their findings, the water is released for consumption, or is restricted from use until it is treated (usually by water production personnel using the reverse osmosis water purification unit [ROWPU]). They also collect water samples for suspect biological agent contamination for supporting medical laboratory analysis (see Appendix B). They conduct medical surveillance activities, to include occupational and environmental health threat surveillance. They conduct limited entomological surveys to determine the existence of disease-vectoring arthropods in the AO. They inspect food service facilities to determine the extent, if any, of NBC contamination. They evaluate the unit's—
The PVNTMED detachment provides PVNTMED services on an area support basis to units within their assigned AO. These services include, but are not limited to—
The US Army Veterinary Service is the Executive Agent for veterinary services to all Services within the DOD. They ensure that food and bottled water supplies are safe and provide veterinary medical and surgical care for government-owned animals throughout the AO. On the integrated battlefield, their role is particularly important; the potential for food supplies becoming contaminated with NBC agents is high. For detailed information on provision of veterinary services see FM 8-10-18.
Food may become contaminated from enemy employment of NBC weapons/agents or from terroristic contamination of food procurement facilities and food supplies. The NBC agents may be introduced during production or in the storage area of the procurement facility; while the product is in transit; at the military storage facility; or at the unit food service facility. Regardless of where the agent is used, the effect is the same; personnel will become ill or die if they consume the contaminated food. To ensure food safety, veterinary personnel inspect and monitor food from its procurement until it is issued to the consumer. Throughout the AO, all Services (Army, Navy, Marine, and Air Force) logistics and food service personnel must take precautions to protect subsistence from contamination.
Veterinary personnel are involved in the detection and monitoring of NBC contaminated rations; before use, they must inspect all food suspected of being contaminated with NBC agents. Appendix J provides guidance on food decontamination procedures. Veterinary personnel provide advice on the decontamination of food to unit personnel owning the food, or personnel performing the food decontamination. Depending on the type of contamination and packaging, the food may be—
Some items may be held to allow time for natural decay of nuclear or chemical contamination before consumption. The commander, with advice from veterinary personnel, makes the decision on the disposition of the food. However, veterinary personnel make the final determination of food safety.
Veterinary personnel are concerned with the protection of government-owned animals and animals being procured for consumption. Animals must be protected from NBC contamination, whenever possible. Animals should be moved into enclosures to protect them as much as possible from contamination. Protective equipment is not available for military working dogs; however, protection of the animal's feet and body must be considered. When military working dogs must cross a contaminated area, protect their feet by using butyl rubber material to improvise booties. Since CPS systems are not available, animal treatment facilities must be established in contamination free areas. Veterinary treatment personnel must remain in MOPP Level 4 when caring for NBC animal casualties until the animals have been decontaminated. The treatment of military working dog NBC casualties is outlined in FM 8-10-18.
Laboratory services must continue their support role even under NBC conditions. For the provision of clinical and diagnostic support, the facility must be located in a contamination-free area or be inside collective protection. Designated laboratories within the theater will analyze NBC samples/specimens (including in theater field confirmation identification of biological agents by evaluating specimens from symptomatic patients and animals and environmental samples collected from the AO). See Appendix B for procedures in collecting biological samples/specimens, handling/packaging, maintaining chain of custody, transporting samples/specimens, and analysis.
Laboratory support at this level is extremely limited; it consists of laboratory procedures in direct support of MTF and FST activities. Laboratory personnel prepare collected suspect NBC specimens for submission to the supporting laboratory for analysis; the specimens are forwarded to supporting medical laboratories (Appendix B).
Laboratory support in a CSH is intended for providing clinical laboratory support and is primarily in support of acute surgical cases, blood services, and statim (STAT) services required for intensive care operations. Only extremely limited microbiology services (parasitological exams and gram stains) are provided. In a mature theater, the microbiology services may be augmented to include limited cultures and sensitivity testing. Patients with documented or suspected exposure to NBC weapons/agents will be medically evaluated, specimens will be collected, packaged, and have chain of custody established. The specimens will be forwarded through technical channels to the supporting medical laboratory (such as the theater Army medical laboratory [TAML]) for analysis. See Appendix B for specimen collection, packaging, chain of custody, and processing requirements.
a. Clinical Laboratories. The clinical laboratories in the combat support, field, and general hospitals have the ability to perform a general, but limited, array of analytical procedures in hematology, urinalysis, chemistry, microbiology, serology, and blood bank. Patient specimens of suspected biological or chemical agent exposures are forwarded through technical channels to the supporting medical laboratory. See Appendix B for sample/specimen collection, packaging, chain of custody, processing, and transporting requirements.
b. Field Laboratories.
(1) Theater Army Medical Laboratory. The TAML is the specialized echelons above corps (EAC) laboratory that provides clinical and nonclinical medical laboratory support. When equipped and staffed, the TAML provides in-theater field confirmation identification of NBC samples or specimens. Using sophisticated equipment and methods, the TAML has the capability to detect and identify NBC agents in a variety of specimens/samples (such as human, air, soil, water, animals, vegetation, and food). Direct support from continental United States (CONUS)-based laboratories aids the TAML with identification of NBC agents. Command decision on use of protective/preventive measures and patient care may be based on the TAML findings. Proper collection, packaging, and rapid shipment of specimens by MTFs and samples from other sources will ensure effective, timely, and accurate laboratory analyses.
(2) Area Medical Laboratory. The Area Medical Laboratory (AML) is the specialized laboratory within the theater that provides nonclinical medical laboratory support. The AML can be deployed in the corps or to EAC for support missions. When fielded, the AML will replace the TAML in the force structure. The AML provides in-theater field confirmation identification of NBC samples or specimens. Using sophisticated equipment and methods, the AML has the capability to detect and identify NBC agents in a variety of specimens/samples (such as human, air, soil, water, animals, vegetation, and food). Direct support from CONUS-based laboratories aids the AML with identification of NBC agents. Command decision on use of protective/preventive measures and patient care may be based on the AML findings. Proper collection, packaging, and rapid shipment of specimens by MTFs and samples from other sources will ensure effective, timely, and accurate laboratory analyses.
Designated Level V medical laboratories perform analyses to provide definitive identification of suspect biological agents for the President and Secretary of Defense purposes. The definitive identification of suspect biological agents also aids commanders in the AO in maintaining the health of their command.
Chemical corps personnel collect environmental, air, soil, and vegetation samples. Preventive medicine personnel collect samples from drinking water sources and supplies. Veterinary personnel collect samples from food supplies and medical specimens from animals. All other units collect soil, vegetation, and small [5-7] animal samples for laboratory analysis. Samples are subjected to initial screening with rapid test kits and in-theater confirmatory identification at the supporting medical laboratory. The President- and Secretary of Defense-required definitive identification is performed at the designated Level V medical laboratory. Comprehensive databases will be maintained to provide historical testing results and will aid in the AO commander's decisions to conduct operations in an NBC environment. See Appendix B for specific procedures for sample collection, packaging, transporting, maintaining chain of custody, and analysis.
Dental service support is provided in the AO at Levels II, III, and IV. Because of their location close to main supply routes and other support assets, dental units are vulnerable to an NBC strike. Nuclear, biological, and chemical operations have an impact at all levels; thus, dental units must be prepared to survive on the integrated battlefield. Defense against NBC weapons must be included in the dental unit's TSOP. Individual and collective tasks must be intensely trained on a regular basis; survival depends on the ability of personnel to use basic survival skills against an NBC attack. For details on provision of dental services, see FM 4-02.19.
The overall mission of dental units to provide dental services is greatly affected in the aftermath of an NBC attack. First, the unit must survive the attack and rapidly recover from its effects. Secondly, in the event of mass casualties, the dental patient care effort must be redirected from dental treatment to the alternate wartime role of augmenting the adjacent MTF. Dental units do not possess CPS; therefore, providing dental services in an NBC environment will be limited to the treatment of maxillofacial emergencies requiring immediate attention. This care will be provided at an MTF with a CPS.
As a general rule, in the aftermath of an NBC attack, dental treatment operations cease until deliberate decontamination of the unit and its equipment has been accomplished. Only maxillofacial injuries of an immediate life-threatening nature should be considered for treatment. After an attack, the resources of the dental treatment facility (DTF) are redirected toward support of any mass casualty situation that may have been generated at an adjacent MTF, or toward decontamination and relocation to a noncontaminated area.
The only category of dental treatment appropriate in an NBC environment is emergency; and then, only those emergencies of an extreme nature which demand immediate attention. The most likely condition requiring such attention would be maxillofacial trauma and would most likely be treated at an MTF rather [5-8] than a DTF. Although the likelihood of a requirement to treat dental patients in an NBC environment is extremely low, DTFs must have a plan in the event that such patients do present.
a. Patient Decontamination. Decontamination of patients, dental patients included, is an absolute requirement before admission into a clean MTF. Contaminated patients are triaged and decontaminated before treatment (except for life- or limb-saving care). Both triage and decontamination should be accomplished as far forward as possible. Specific details on patient decontamination are in Appendix G. It is important to note that normally patient decontamination is not performed by medical or dental personnel. Initial decontamination at the basic skill level is accomplished at the casualty's unit. Detailed patient decontamination is accomplished by the patient decontamination teams (made up of nonmedical personnel from the supported units) that are supervised by medical personnel at the MTF.
b. Patient Decontamination at Dental Treatment Facilities. Neither dental units nor their DTFs are equipped for patient decontamination. Any contaminated patients arriving at a DTF requiring urgent attention must be directed or evacuated to the nearest MTF with a patient decontamination capability.
Dental treatment facilities must also consider the need to protect patients in their care in the event of an NBC attack, or when the threat of an attack is high. Special consideration must be made for maxillofacial patients whose condition prevents them from wearing their protective mask.
a. Immediate Response. In the event of an attack or when the alarm sounds, dental treatment providers immediately cease work and mask. The patients should do likewise. Only after putting on their own masks, do the dental treatment providers assist the patient, if necessary, by removing materials that impede the patient's masking. Only those materials that impede masking or may compromise the airway (such as rubber dam frames or impressions) are removed, the rest are left in place until the all clear is sounded. Special attention must be given to patients who may have been medicated into a less than fully conscious state, or are otherwise incapacitated.
b. Mission-Oriented Protective Posture Considerations. The MOPP level should be taken into account when determining the category and extent of dental treatment to be provided. Patients, including those seated in the dental chair, should be at the MOPP level prescribed for the DTF by its parent headquarters. Dental treatment at MOPP Levels 3 and 4 is, of course, impossible because of the requirement to wear the protective mask; however, treatment is still possible at MOPP Levels 0, 1, and 2. Treatment at MOPP Level 2 should be limited only to emergency care requiring urgent attention. At MOPP Level 1, most types of dental emergencies can be accommodated; however, only minimal essential treatment should be undertaken in order to reduce risk of the patient being caught in a compromised state. At MOPP Level 0, the provision of dental treatment generally is not limited. However, the degree of the NBC threat forecast for the area should be considered before undertaking extensive treatment.
c. Maxillofacial Injuries. Patients with maxillofacial injuries that prevent proper fit and seal of the individual protective mask must be placed in a PPW. Though patients with these types of injuries are [5-9] most likely to be found only in MTF channels, DTFs should nevertheless be prepared in the event a patient presents to the DTF. Since the DTF does not have any PPWs; these patients should be immediately evacuated to the adjacent MTF for treatment.
When operating under the threat of or under actual NBC conditions, soldiers will be at a high risk of suffering combat operational stress-related conditions. The invisible, pervasive nature of these weapons creates a higher degree of uncertainty and ambiguity, presenting fertile opportunities for false alarms, mass panic, and other maladaptive stress reactions. Therefore, commanders and leaders must take actions to prevent and reduce the numbers of combat operational stress cases in this environment. The symptoms and physical signs caused by excessive stress are similar to some signs of true NBC agent injury. In World War I, inexperienced units initially evacuated two stress cases for every one true chemical casualty. Some minor chemical casualties also had major stress symptoms. Therefore, far forward triage is essential to prevent over evacuation and loss of the individual to the unit. For details on provision of COSC see FM 8-51 and FM 22-51.
a. Keep Personnel Informed of the Situation. Keep information flowing, dispel myths, and control rumors by—
b. Train Soldiers to Survive. Use training procedures that—
[5-10] c. Put Nuclear, Biological, and Chemical Defense in Realistic Perspective. Continuously strive to maintain a realistic perspective in the unit by—
d. Train in the Protective Mask. Train in the protective mask often. It takes repeated wear and time to acclimate and get over the claustrophobic feeling of wearing the mask. The training can be conducted during a variety of activities.
e. Train in Mission-Oriented Protective Posture Level 4. Training in MOPP Level 4 (or simulated MOPP 4, which is to overdress while wearing the protective mask, overboots, and gloves) will increase personnel confidence in their ability to wear the ensemble.
f. Ensure Sleep Plans are Safely Practiced. Have everyone practice wearing the mask while sleeping. Ensure personnel only sleep in safe places; do not allow personnel to sleep under or near vehicles or other motorized machinery. Require ground guides for all vehicles in the unit bivouac area. Ensure that each individual get at least 4 hours of uninterrupted sleep during every 24-hour period, mission permitting (See FM 21-10).
a. Follow Orders. By following orders, individuals can increase their ability to cope with and prevent combat operational stress-related conditions. Coping with the stresses of an NBC environment requires extra individual action. Concentrate on the positive aspects of survival, not the negatives of illness or death.
[5-11] b. Train. Use every opportunity to wear the protective mask or the entire MOPP ensemble during training, when permitted. You build self-confidence and endurance by frequently training with your protective mask, or at MOPP Level 4. Perform refresher training in basic NBC survival skills.
c. Use Buddy System. Use the buddy system to increase your ability to survive. Service members looking out for each other give a sense of security that relieves stress. Looking out for each other improves every individual's ability to perform his duties.
a. Staffing for Combat Operational Stress Control. Combat operational stress control is provided by the following activities or units:
b. Conduct Preventive Activities. In an NBC environment, prevention is the most economical means of controlling combat operational stress reactions. Mental health personnel must begin consultation services before NBC weapons/agents have been employed.
c. Control Stress Reactions. Individuals with combat operational stress reactions require prompt intervention. The evaluation of over-stressed personnel is difficult but not impossible when both the soldier and the evaluator are in MOPP. The primary method of mental health evaluation is the interview and mental status examination. For details on controlling stress reactions, see FM 8-51.
As in all combat situations, the protection of medical supplies and equipment on the integrated battlefield is a must. Without medical supplies and equipment, HSS will be greatly diminished. Thus, the flow of [5-12] supplies must continue to forward units as they are requested, including during NBC operations. For detailed information on providing health service logistics see FM 4-02.1 and FM 8-10-9.
Protecting supplies can be accomplished by placing them under tents, using plastic wraps, or providing storage warehouses with CB filtered-conditioned (heated or cooled) air systems. Wrapping supplies in two layers of plastic material provides protection from most agents for a short period of time; the thicker the plastic material, the longer the protection. Effectiveness of protective procedures can be checked by placing M9 tape on supplies and between layers of the covering. Protection from the thermal and blast effects of nuclear detonations requires much more elaborate measures. Placing the supplies in trenches, inside earthen berms, behind stonewalls, or in other field expedient facilities will enhance the protective posture of supplies from the nuclear effects. Even when taking these protective measures, a quantity of supplies will become contaminated and must be replaced. Plans should be in place for replacement of lost items.
During shipment, supplies are protected by placement inside MILVANs, in covered enclosed vehicles, or by wrapping them in several layers of plastic, in tarpaulins, or in other protective material. To monitor exposure of supplies to chemical agents during shipment, place M9 detector paper between the wrappings. If exposure is limited to the outer layer, simple removal of this layer may be all that is required to eliminate the contamination. Decontamination is much easier when the supplies and equipment have been protected by multilayers of over-wraps.
Maintenance on vehicles, equipment, and medical equipment will become much more complex under NBC conditions. Most chemical agents are soluble in organic solvents such as gasoline, motor oils, and lubricants. The agent may be removed from the equipment by these solvents, but exposure to the contaminated solvents will produce the same effects as exposure to the agent on the equipment. The agents may seep down around the threads of bolts, in cracks and crevices of the equipment, and inside the cabinets or enclosures of equipment. These potential contamination sources produce an increased hazard to maintenance personnel. Decontamination of some items, especially medical equipment, may be a problem for maintenance personnel. The use of standard decontamination agents will cause damage beyond repair to most medical equipment and electronic equipment. In some instances, removal of chemical agents will require aging (off-gassing) of the agent. Turning the equipment on and running it, or just exposing the equipment to warm air will speed the off-gassing process. Maintenance personnel must perform all procedures in MOPP Level 4 until decontamination is completed. Radiation will penetrate the metal structures of vehicles and other equipment; radioactive material will be absorbed into the lubricants and fuels. Decontamination of this type of contamination is very difficult, if not impossible. Personnel must use radiation detection equipment to determine the extent of contamination and decontaminate the items as much as possible. Dusting or washing with water can remove any fallout on the surface of vehicles and nonelectrical/electronic [5-13] components of equipment. Removal of radioactivity absorbed into metals or mixed in lubricants and fuels is beyond the capabilities of unit personnel. See FM 3-5 for decontamination procedures.
Although, homeland security is not a specific military mission, commanders must plan for and be prepared to support a lead federal agency (such as the Federal Bureau of Investigation or Federal Emergency Management Agency) in response to CBRNE event. When the CBRNE event occurs on a military installation, the Weapons of Mass Destruction—Incident Support Team (WMD-IST) is the lead federal agency in charge of responding and establishes an incident command center (ICC). The installation medical authority (IMA) provides the HSS initial response to the event site. Request for assistance from deployable HSS organizations and staffs are initiated by the IMA through military channels. The incident commander will submit a request for HSS assistance to a CBRNE event off the military installation through the appropriate federal channels. The President will direct any DOD response in support of a lead federal agency to a CBRNE event. The Presidential direction to assist will be passed down through military channels to the appropriate HSS organization for response. The HSS response may be in the form of special medical augmentation teams (SMART) support from US Army Medical Command resources or HSS (table of organization and equipment [TOE]) units may be directed to respond. Normally, responding TOE units will provide HSS to nonmedical military responders. However, the HSS mission may be to provide support to the lead federal agency or civilian public health organizations, emergency medical services (ambulance crews), or hospitals. The HSS response will include, but not be limited to—
For detailed Information on capabilities of SMARTs see FM 4-02 and FM 8-42. For detailed information on capabilities and functions of TOE HSS units see FM 4-02- and 8-series publications.
Biological and chemical weapons/agents may be employed by assassins, terrorists, rebels, and insurgents, as well as well-formed battle organizations, across the continuum of operations. In addition, nuclear weapons will remain a threat on the future battlefield. Another weapon that may be used is the RDD. The RDD can cause significant damage and present health hazards to fighting forces by exposing them to radiation without the thermal and full blast effects of nuclear weapons. The RDD can disperse radioactive material over an area of the battlefield; the area covered is dependent upon the amount of radioactive and explosive material used. In order to detonate a nuclear weapon, an adversary must first obtain access to the appropriate weapons-grade material. However, an RDD can be produced and used by anyone with access to industrial or medical radioisotopes and explosives. Biological agents are easy to disperse on the battlefield without immediate detection; however, their effects on exposed troops can change the course of the battle. Some nations consider chemical weapons as a component of their munitions for the battlefield. As more nations enter the arena of developing biological and chemical weapons, their potential effects on our troops will increase. The enemy's use of TIMs as weapons or collateral damage to TIM storage faculties can severely affect the unit personnel's ability to continue the mission. The signs and symptoms of some TIM exposure can be the same as those presented from exposure to NBC weapons. Considerations of both the physical and biological effects of these weapons are required for HSS operations. Field Manual 4-02.283 provides additional information on nuclear and radiological effects; FM 8-284 provides additional information on biological agent effects; FM 8-285 provides additional information on CW effects; FM 8-500 provides detailed information on hazardous material (TIM) effects.
a. The principal physical effects of nuclear weapons are blast, thermal radiation (heat), and nuclear radiation. These effects are dependent upon the yield (or size) of the weapon expressed in kilotons (KT), the physical design of the weapon (such as conventional and enhanced), and the method of employment. The distribution of energy (Figure A-1) from the detonation of a moderate-sized (3 to 10 KT) weapon is as follows:
(1) Fifty percent as blast.
(2) Thirty-five percent as thermal radiation; made up of a wide spectrum of electromagnetic radiation, including infrared, visible, and ultraviolet light and some soft x-ray radiation.
(3) Fourteen percent as nuclear radiation, 4 percent as initial ionizing radiation composed of neutrons and gamma rays emitted within the first minute after detonation, and 10 percent as residual nuclear radiation (fallout).
(4) One percent as EMP.
b. Larger weapons are more destructive than smaller weapons, but the destructive effect is not linear. Table A-1 presents a comparison of three aspects of nuclear weapons effects with yield.
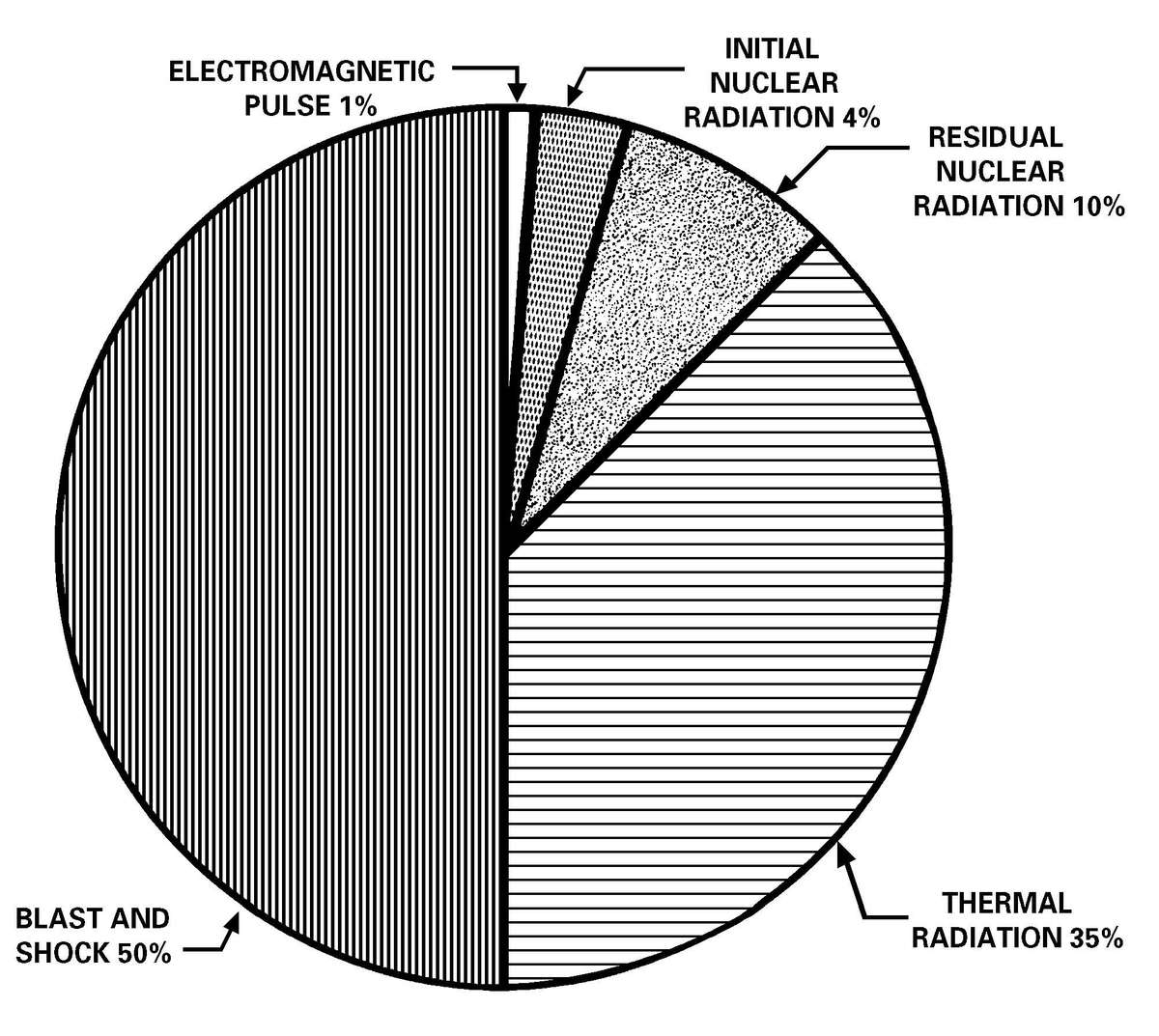
Figure A-1. Distribution of energy.
c. The altitude at which the weapon is detonated determines the blast, thermal, and nuclear radiation effects. Nuclear blasts are classified as air, surface, or subsurface bursts.
(1) An airburst is a detonation in air at an altitude below 30,000 meters, but high enough that the fireball does not touch the land or water surface. The altitude is varied to obtain the desired tactical effects. Initial radiation will be a significant hazard, but there is essentially no local fallout. However, the ground immediately below the airburst may have a small area of neutron-induced radioactivity. This may pose a hazard to troops passing through the area.
(2) A surface burst is a detonation in which the fireball actually touches and vaporizes the land or water surface. In this case, the area affected by blast, thermal radiation, and initial nuclear radiation will be smaller than for an airburst of comparable yield. However, in the region around ground zero, the [A-3] destruction will be much greater and a crater is often produced. Additionally, all the material that was within the fireball becomes fallout and will be a hazard downwind. A surface burst is the most likely type of terrorist detonation.
(3) A subsurface burst is an explosion in which the detonation is below the surface of land or water. Cratering usually results. If the burst does not penetrate the surface, the only hazard is from the ground or water shock. If the burst penetrates the surface, blast, thermal, and initial nuclear radiation will be present, though less than for a surface burst of comparable yield. Local fallout will be heavy over a small area.
(4) A high altitude burst occurs above 30,000 meters. Radiation and physical effects do not reach the ground and there is no local fallout. This is the only detonation where the effects of the EMP are significant. Nonhardened electronic equipment including many medical devices may become inoperative. The EMP damage is a moot point with other types of detonations, as its range is primarily limited to the area of intense physical destruction.
Table A-1. Comparison of Weapons Effects (Radii of Effects in Kilometers—Airburst)
| 1 KT | 20 KT | 100 KT | 1 MT | 10 MT | |
|---|---|---|---|---|---|
| NUCLEAR RADIATION (1,000 cGy) |  | ||||
| BLAST (50% INCIDENCE OF TRANSLATION WITH SUBSEQUENT IMPACT WITH A NON-YIELDING SURFACE) |  | ||||
| THERMAL (50% INCIDENCE OF 2ND-DEGREE BURNS TO BARE SKIN, 10 KM VISIBILITY) |  | ||||
The physiological effects of nuclear weapons are the result of exposure to the blast; thermal radiation; ionizing radiation (initial or residual) effects; or a combination of these. For smaller weapons (less than 10 KT), ionizing radiation is the primary creator of casualties requiring medical care, while for larger weapons (greater than 10 KT), thermal radiation is the primary creator of casualties.
a. The rapid compression and decompression of blast waves on the human body results in transmission of pressure waves through the tissues. Resulting damage is primarily at junctions between tissues of different densities (bone and muscle), or at the interface between tissue and airspace. Lung tissue and the gastrointestinal system (both contain air) are particularly susceptible to injury. The tissue disruptions can lead to severe hemorrhage or to an air embolism; either can be rapidly fatal. Direct overpressure effects do not extend out as far from the point of detonation as the drag force and are often masked by the drag force effects. A typical range of probability of lethality, with variations in overpressure for a 1 KT weapon, is shown in Table A-2.
Table A-2. Range of Lethality of Peak Overpressure
| LETHALITY (APPROXIMATE %) | PEAK OVERPRESSURE (ATMOSPHERES) | DISTANCE FROM GROUND ZERO; METERS |
|---|---|---|
| 1 | 2.3-2.9 | 150 |
| 50 | 2.9-4.1 | 123 |
| 100 | 4.1+ | 110 |
(1) The significance of the data is that the human body is relatively resistant to static overpressure compared to rigid structures such as buildings. For example, an unreinforced cinder block panel will shatter at 0.1 to 0.2 atmospheres.
(2) Overpressure lower than those in Table A-2 can cause nonlethal injuries such as lung damage and eardrum rupture. Lung damage is a relatively serious injury, usually requiring hospitalization, even if not fatal; whereas eardrum rupture is a minor injury, often requiring no treatment at all.
(a) The threshold level of overpressure for an unreinforced unreflected blast wave that can cause lung-damage is about 1.0 atmosphere.
(b) The threshold level for eardrum rupture is around 0.2 atmospheres; the overpressure associated with a 50 percent probability of eardrum rupture is about 1.1 atmospheres.
(3) Casualties requiring medical treatment from direct blast effects are produced by overpressure between 1.0 and 3.5 atmospheres. However, other effects (such as indirect blast injuries and thermal injuries) are so predominate that patients with only direct blast injuries make up a small part of the patient workload.
b. The drag forces (indirect blast) of the blast winds are proportional to the velocities and duration of the winds. The winds are relatively short in duration, but can reach velocities of several hundred km per hour. Injury can result from missiles impacting on the body or from the physical displacement of the body against objects and structures.
(1) The distance from the point of detonation at which severe indirect injury occurs is greater than that for equally serious direct blast injuries. A high probability of serious indirect injury can occur when the peak overpressure is about 0.2 atmospheres. This range will increase with the increased size of the weapon; for a 1 KT weapon, the range is 0.22 km, whereas for a 20 KT weapon, the range is 0.76 km. At greater ranges injuries will occur and casualties will be generated, but not consistently.
(2) The drag forces of the blast winds produced by a nuclear detonation are so great that almost any form of vegetation or structure will be broken up or fragmented into missiles. Thus, multiple, varied missile injuries will be common, increasing their overall severity and significance. Table A-3 lists ranges at which significant missile injuries can be expected.
Table A-3. Ranges for Probabilities of Serious Injury from Small Missiles
| RANGES (km) | |||
|---|---|---|---|
| YIELD (KT) | 1% PROBABILITY OF SERIOUS INJURY | 50% PROBABILITY OF SERIOUS INJURY | 99% PROBABILITY OF SERIOUS INJURY |
| 1 | 0.28 | 0.22 | 0.17 |
| 10 | 0.73 | 0.57 | 0.44 |
| 20 | 0.98 | 0.76 | 0.58 |
| 50 | 1.4 | 1.1 | 0.84 |
| 100 | 1.9 | 1.5 | 1.1 |
| 200 | 2.5 | 1.9 | 1.5 |
| 500 | 3.6 | 2.7 | 2.1 |
| 1,000 | 4.8 | 3.6 | 2.7 |
| 1 | INCIDENCE OF INJURY BASED ON SKIN AND TISSUE PERFORATION. | ||
| 2 | MISSILES USED WERE 10 GRAM (gm) IN WEIGHT. | ||
(3) The velocity to which missiles are accelerated is the major factor in causing injury. The probability of a penetration injury increases with increasing velocity, particularly for small, sharp missiles such as glass fragments. Small, light objects are accelerated to speeds approaching the maximum (wind) velocity. Table A-4 shows data for probability of penetration related to size and velocity of glass fragments.
Table A-4. Probability of Glass Fragments Penetrating the Abdominal Cavity
| MASS OF GLASS FRAGMENTS (gm) | 1% | 50% | 99% |
|---|---|---|---|
| IMPACT VELOCITY (METERS PER SECOND) | |||
| 0.1 | 78 | 136 | 243 |
| 0.6 | 53 | 91 | 161 |
| 1.0 | 46 | 82 | 143 |
| 10.0 | 38 | 60 | 118 |
(4) Heavy, blunt missiles may not penetrate, but can result in significant injury, particularly fractures. The threshold velocity for skull fractures from a 4.5 milligram (mg) missile is about 4.6 meters per second (m/sec).
(5) The drag forces of the blast winds are strong enough to displace large objects (such as vehicles), or cause large structures to collapse (such as buildings) resulting in serious crushing injuries. Man himself can become a missile resulting in injuries (called translational injuries). The velocity at which the body is displaced will determine the probability and the severity of injury. Assuming a displacement of 3.0 meters, the impact velocity associated with various degrees of injury is shown in Table A-5. The velocities in Table A-5 can be correlated against yield. The ranges at which such velocities can occur and the probability of injury are given in Table A-6.
Table A-5. Translational Injuries
| A. | BLUNT INJURIES AND FRACTURES | |||
|---|---|---|---|---|
| PROBABILITY OF INJURY | VELOCITY (m/sec) | |||
| 1% | 2.6 | |||
| 50% | 6.6 | |||
| 99% | 16.5 | |||
| B. | FATAL INJURIES | |||
| PROBABILITY OF FATALITY | VELOCITY (m/sec) | |||
| 1% | 6.6 | |||
| 50% | 17.0 | |||
| 99% | 39.7 | |||
Table A-6. Ranges for Selected Impact Velocities of a 70-Kilogram Human Body Displaced by Blast Wind Drag Forces for Different Yield Weapons
| WEAPON YIELD (KT) | VELOCITIES (m/sec) | ||
|---|---|---|---|
| 2.6 | 6.6 | 17.0 | |
| RANGES (km) | |||
| 1 | 0.38 | 0.27 | 0.19 |
| 10 | 1.0 | 0.75 | 0.53 |
| 20 | 1.3 | 0.99 | 0.71 |
| 50 | 1.9 | 1.4 | 1.0 |
| 100 | 2.5 | 1.9 | 1.4 |
| 200 | 3.2 | 2.5 | 1.9 |
| 500 | 4.6 | 3.6 | 2.7 |
| 1,000 | 5.9 | 4.8 | 3.6 |
The thermal radiation emitted by a nuclear detonation causes burns in two ways—by direct absorption of the thermal energy through exposed surfaces (flash burns); or by the indirect action of fires in the environment (flame burns). Indirect flame burns can easily outnumber all other types of injury.
a. Thermal radiation travels outward from the fireball in a straight line; therefore, the amount of energy available to cause flash burns decreases rapidly with distance. Close to the fireball all objects will be incinerated. The range for 100 percent lethality will vary with yield, height of burst, weather, environment, and immediacy of treatment. The critical factors determining the degree of burn injury are the flux (calories per square centimeter/second [cal/cm2/sec]) and the duration of the thermal pulse. The total amount of thermal radiation needed to cause a flash partial thickness burn on exposed skin will vary with the yield of the weapon and the nature of the pulse (Table A-7). Most burn patients will come from the zones where partial thickness burns occur. In areas where radiation, blast, and thermal intensity are highest, burn victims surviving long enough to reach medical care will be rare.
NOTE
The battle dress uniform (BDU), MOPP gear, or any other clothing will provide additional protection against flash burns. The airspace between the clothing significantly impedes heat transfer and may prevent or reduce the severity of burns, depending on the magnitude of the thermal flux.
Table A-7. Factors for Determining the Probability of Partial Thickness Burns
| YIELD OF WEAPON | 1 KT | 10 KT | 100 KT | 1 MT | 10 MT |
|---|---|---|---|---|---|
RANGE (km) FOR PRODUCTION OF PARTIAL THICKNESS BURNS ON EXPOSED SKIN | 0.78 | 2.1 | 4.8 | 9.1 | 14.5 |
DURATION OF THERMAL PULSE IN SECONDS | 0.12 | 0.32 | 0.9 | 2.4 | 6.4 |
Cal/cm2/sec REQUIRED TO PRODUCE PARTIAL THICKNESS BURNS ON EXPOSED SKIN | 4.0 | 4.5 | 5.3 | 6.3 | 7.0 |
b. Indirect (flame) burns result from exposure to fires caused by the thermal effects in the environment, particularly from ignition of clothing. The larger-yield weapons are more likely to cause firestorms over extensive areas. There are too many variables in the environment to predict either incidence or severity of casualties. Expect the burns to be far less uniform (in degree) and not limited to exposed [A-8] surfaces. For example, the respiratory system may be exposed to the effects of hot gases produced by extensive fires. Respiratory system burns cause high morbidity and high mortality rates.
c. The initial pulse of radiation in the optical and thermal bands can cause injuries in the forms of flash blindness and retinal scarring. The initial brilliant flash of light produced by the nuclear detonation causes flash blindness. This flash swamps the retina, bleaching out the visual pigments and producing temporary blindness. During daylight hours, this temporary effect may last for about 2 minutes. At night, with the pupil dilated for dark adaptation, flash blindness will affect personnel at greater ranges and for greater durations. Partial recovery can be expected in 3 to 10 minutes, though it may require 15 to 35 minutes for full night adaptation recovery. Retinal scarring is the permanent damage from a retinal burn. It will occur only when the fireball is actually in the individual's field of view and should be a relatively uncommon injury. The location of the scar will determine the degree of interference with vision. Because night vision apparatus electronically amplifies an image, it cannot transmit the flash intensity and will not cause eye injury.
A nuclear burst results in four types of ionizing radiation: neutrons, gamma rays, beta, and alpha radiation. The initial burst is characterized by neutrons and gamma rays while the residual radiation is primarily alpha, beta, and gamma rays. The effect of radiation on a living organism varies greatly by the type of radiation to which the organism is exposed. See Table A-8 for characteristics of nuclear radiation.
a. Alpha particles are extremely massive, charged particles (four times the mass of a neutron); they are a fallout hazard. Because of their size, alpha particles cannot travel far and are fully stopped by the dead layers of the skin or by the uniform. Alpha particles are a negligible external hazard, but if inhaled or ingested, can cause significant internal damage.
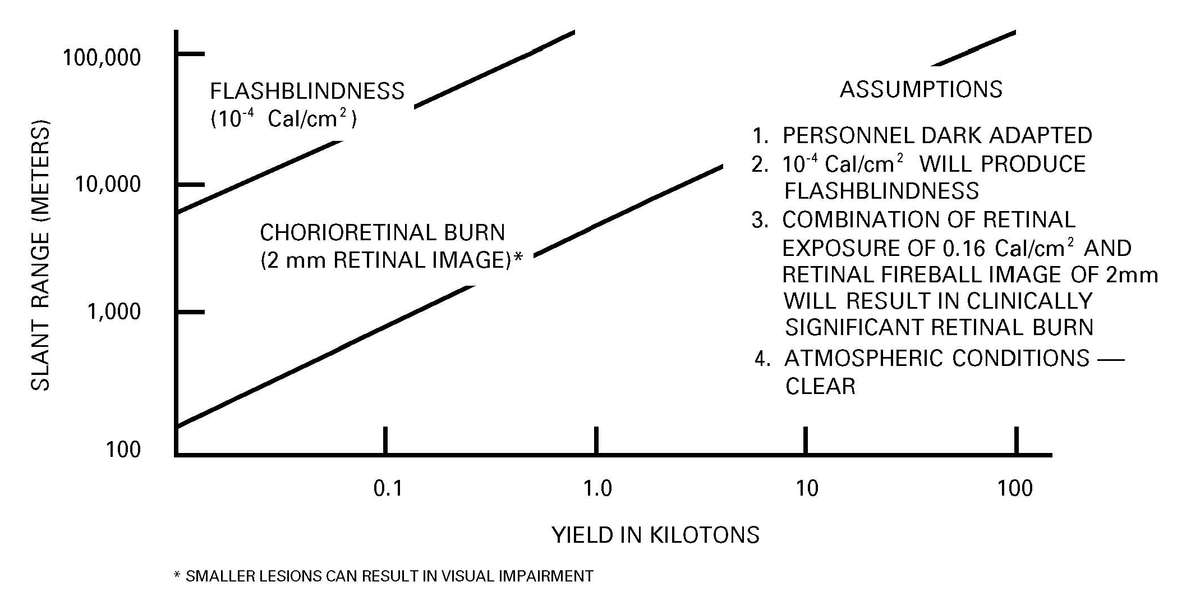
Figure A-2. Threshold distance for minimal chorioretinal burn and flash blindness versus yield (airburst) at night.
Table A-8. Characteristics of Nuclear Radiation
| NAME AND SYMBOL | WHAT IS IT | SOURCE | ENERGY AND SPEED | RANGE IN AIR | RANGE IN TISSUE | SHIELDING REQUIRED | BIOLOGICAL HAZARD |
| α ALPHA PARTICLE | HELIUM NUCLEUS  |
DECAY OF URANIUM AND PLUTONIUM | ENERGY VARIES: SPEED VARIES FROM 1/20 TO 1/10 SPEED OF LIGHT | ~ 5 cm | CANNOT PENETRATE THE EPIDERMIS | NONE | NONE, UNLESS INGESTED OR INHALED IN SUFFICIENT QUANTITIES |
| β BETA PARTICLE | HIGH-SPEED SPEED ELECTRON  |
DECAY OF FISSION PRODUCTS AND NEUTRON INDUCED ELEMENTS | VARIES OF SKIN | 5 METERS OR MODERATE | SEVERAL LAYERS SKIN INJURY | STOPPED BY A FEW cm OF Al CLOTHING | SUPERFICIAL |
| γ GAMMA RAY | ELECTRO-MAGNETIC ENERGY  |
DECAY OF FISSION PRODUCTS AND NEUTRON INDUCED ELEMENTS | ENERGY VARIES: TRAVELS AT THE SPEED OF LIGHT | UP TO 500 METERS, BUT IS ENERGY DEPENDENT | VERY PENETRATING, BUT IS ENERGY DEPENDENT | DENSE MATERIAL, SUCH AS CONCRETE, STEEL PLATE, EARTH | WHOLE BODY INJURY, MANY CASUALTIES POSSIBLE |
| η NEUTRON | UNCHARGED PARTICLE  |
FISSION AND FUSION REACTIONS | VARIES | LESS THAN GAMMA, BUT IS ENERGY DEPENDENT | VERY PENETRATING, BUT IS ENERGY DEPENDENT | HYDROGENOUS MATERIALS, SUCH AS WATER OR DAMP EARTH | WHOLE BODY INJURY, MANY CASUALTIES POSSIBLE |
b. Beta particles are very light, charged particles that are found primarily in fallout radiation. These particles can travel a short distance in tissue; if large quantities are involved, they can produce damage to the basal stratum of the skin. The lesion produced is similar to a thermal burn (called a beta burn).
c. Gamma rays, emitted during the nuclear detonation and in fallout, are uncharged radiation similar to X rays. They are highly energetic and pass through matter easily. Because of its high penetrability, radiation can be distributed throughout the body, resulting in whole body exposure.
d. Neutrons, like gamma rays, are uncharged, are only emitted during the nuclear detonation, and are not a fallout hazard. However, neutrons have significant mass and interact with the nuclei of atoms, severely disrupting atomic structures. Compared to gamma rays, they can cause 20 times more damage to tissue.
e. When radiation interacts with atoms, energy is deposited resulting in ionization (electron excitation). This ionization may involve certain critical molecules or structures in a cell, producing its characteristic damage. Two modes of action in the cell are direct and indirect action. The radiation may directly hit a particularly sensitive atom or molecule in the cell. The damage from this is irreparable; the cell either dies or is caused to malfunction. The radiation can also damage a cell indirectly by interacting with water molecules in the body. The energy deposited in the water leads to the creation of toxic molecules; the damage is transferred to and affects sensitive molecules through this toxicity.
f. The most radiosensitive organ systems in the body are the male reproductive, the hematopoietic, and the gastrointestinal systems. The relative sensitivity of an organ to direct radiation injury depends upon its component tissue sensitivities. Cellular effects of radiation, whether due to direct or indirect damage, are basically the same for the different kinds and doses of radiation. The simplest effect is cell death. With this effect, the cell is no longer present to reproduce and perform its primary function. Changes in cellular function can occur at lower radiation doses than those that cause cell death. Changes can include delays in phases of the mitotic cycle, disrupted cell growth, permeability changes, and changes in motility. In general, actively dividing cells are most sensitive to radiation. Additionally, radiosensitivity tends to vary inversely with the degree of differentiation of the cell.
g. Predicting radiation effects is difficult because often it is unknown which organs were exposed. Thus, most predictions are based on whole body irradiation. Partial body and specific organ irradiation will occur due to shielding by equipment, from fallout particles, or from internal deposition. Depending upon the organ system, the irradiation can be severe. The severe radiation sickness resulting from external, whole body irradiation and its consequent organ effects is a primary medical concern. The median lethal dose (LD) of radiation that will kill 50 percent of the exposed persons within a period of 60 days (designated as LD50/60) is estimated to be approximately 4.5 gray (Gy) if appropriate medical care is not provided to the casualties. Medical intervention should raise this figure to approximately 10 Gy. This larger figure includes most of the casualties who would be actually capable of reaching medical care following a nuclear detonation, and nearly all those who could be exposed to a RDD. For acute effects of single high dose rate exposures of whole-body irradiation to healthy adults see Table A-9.
h. Recovery of a particular cell system will occur if a sufficient fraction of a given stem cell population remains after radiation injury and appropriate stimulation and protection are received. Complete recovery may appear to occur; however, the immune system may repair incompletely with consequent greater susceptibility to future insult from a variety of agents. It is possible for late somatic effects to have a higher probability of occurring because of the radiation damage. Efficacy of both prior and future immunization in this group is not adequately understood.
i. Interactions between radiological injury and chemical or biological agents appear to be synergistic. Insult by these agents in radiologically injured personnel, even in individually subclinical dosages, may result in significant clinical illness.
a. Radiologically Contaminated Patients. Personnel from contaminated areas may have fallout on their skin and clothing. Although the individual will not be radioactive, he may suffer radiation injury from the contamination. Removal of the contamination should be accomplished as soon as possible; definitely before admission into a clean treatment area. The distinction must be made between a radiation-injured soldier and one who is radiologically contaminated. Although personnel may have received substantial radiation exposure, this exposure alone does not result in the individual being contaminated. Contaminated personnel do not pose a short-term hazard to the medical staff, rather the contamination is a hazard to the individuals' health. However, without patient decontamination, medical personnel may receive sufficient exposure to create beta burns, especially with extended exposure.
b. Handling Radiologically Contaminated Patients. To properly handle radiologically contaminated personnel, medical personnel must first detect the contamination. Detectors that may be used are the AN/PDR27 and AN/VDR2 to monitor patients for contamination. Generally, a reading on the meter twice the current background reading indicates that the patient is contaminated. Monitoring is conducted when potentially contaminated personnel arrive at the MTF. This monitoring is conducted at the MTF's receiving point before admitting the patient. Contaminated patients must be decontaminated before admission. Removal of radiological contamination is less important than immediate lifesaving treatment and providing the best possible medical care. Lifesaving care before decontamination is provided outside the MTF.
c. Decontamination. Removing all outer clothing and a brief washing or brushing of exposed skin will reduce 95 percent of contamination; vigorous bathing or showering is unnecessary. See Appendix G for patient decontamination procedures.
d. Internal Contamination. Internalization of radioactive isotopes will primarily occur via inhalation, ingestion, and contaminated wounds. Extensive internal decontamination should only be undertaken when individual dose estimates indicate that the individual will benefit from the procedures. Soldiers who wear their protective mask will be adequately protected from inhalation and ingestion of radioactive particulate matter. Internal contamination is considered a delayed problem and does not influence triage categories, as does irradiation injury.
e. Treatment. Treatment procedures for radiation injuries are described in FM 4-02.283, FM 8-9, and the NATO Handbook, Emergency War Surgery. Appropriate medical intervention and bone marrow resuscitation will prevent most deaths secondary to irradiation and infection.
Table A-9. Acute Clinical Effects of Single High Dose Rate Exposures of Whole-body Irradiation of Healthy Adults
[Part 1]
| DOSE (RANGE) | 0-100 cGy (SUBCLINICAL RANGE) | 100-1000 cGy (SUBLETHAL RANGE) | ||||
| 100-200 cGy | 200-600 cGy | 600-1000 cGy | ||||
| INITIAL PHASE | INCIDENCE OF NAUSEA & VOMITING | NONE | 5-50% | 50-100% | 75-100% | |
| TIME OF ONSET | —— | APPROX 3-6 HRS | APPROX 2-4 HRS | APPROX 1-2 HRS | ||
| DURATION | —— | LESS THAN 24 HRS | LESS THAN 24 HRS | LESS THAN 48 HRS | ||
| COMBAT EFFECTIVENESS | 100% | 100% | CAN PERFORM ROUTINE TASKS. SUSTAINED COMBAT OR COMPARABLE ACTIVITIES HAMPERED FOR 6-20 HRS. | CAN PERFORM ONLY SIMPLE ROUTINE TASKS. SIGNIFICANT INCAPACITATION IN UPPER PART OF RANGE. LASTS MORE THAN 24 HRS. | ||
| LATENT PHASE | DURATION | —— | MORE THAN 2 WEEKS | APPROX 7-15 DAYS | NONE TO APPROX 7 DAYS | |
| SECONDARY PHASE | SIGNS & SYMPTOMS | NONE | MODERATE LEUKOPENIA | SEVERE LEUKOPENIA; PURPURA, HEMORRHAGE; INFECTION; EPILATION ABOUT 300 cGy. | ||
| TIME OF ONSET POST EXPOSURE | —— | 2 WEEKS OR MORE | SEVERAL DAYS TO 2 WEEKS | |||
| CRITICAL PERIOD POST EXPOSURE | —— | NONE | 4-6 WEEKS | |||
| ORGAN SYSTEM RESPONSIBLE | NONE | HEMATOPOIETIC TISSUE | ||||
| HOSPITAL-IZATION | PERCENTAGE | NONE | LESS THAN 5% | 90% | 100% | |
| DURATION | —— | 45-60 DAYS | 60-90 DAYS | 90-120 DAYS | ||
| INCIDENCE OF DEATH | NONE | NONE | 0-80% | 90-100% | ||
| AVERAGE TIME OF DEATH | —— | —— | 3 WEEKS TO 2 MONTHS | |||
| THERAPY | NONE | REASSURANCE HEMATOLOGIC SURVEILLANCE | BLOOD TRANSFUSION, ANTIBIOTICS | |||
[Part 2]
| DOSE (RANGE) | OVER 1000 cGy (LETHAL RANGE) | |||
| 1000-3000 cGy | OVER 3000 cGy | |||
| INITIAL PHASE | INCIDENCE OF NAUSEA & VOMITING | 100% | ||
| TIME OF ONSET | LESS THAN 1 HR | |||
| DURATION | LESS THAN 48 HRS | APPROX 48 HRS | ||
| COMBAT EFFECTIVENESS | PROGRESSIVE INCAPACITATION FOLLOWING AN EARLY CAPABILITY FOR INTERMITTENT HEROIC RESPONSE. | PROGRESSIVE INCAPACITATION FOLLOWING AN EARLY CAPABILITY FOR INTERMITTENT HEROIC RESPONSE. | ||
| LATENT PHASE | DURATION | NONE TO APPROX 2 DAYS | NONE | |
| SECONDARY PHASE | SIGNS & SYMPTOMS | DIARRHEA; FEVER; DISTURBANCE OF ELECTROLYTE BALANCE. | CONVULSIONS; TREMOR ATAXIA; LETHARGY. | |
| TIME OF ONSET POST EXPOSURE | 2-3 DAYS | |||
| CRITICAL PERIOD POST EXPOSURE | 5-14 DAYS | 1-48 HRS | ||
| ORGAN SYSTEM RESPONSIBLE | GASTROINTESTINAL TRACT | CENTRAL NERVOUS SYSTEM | ||
| HOSPITAL-IZATION | PERCENTAGE | 100% | 100% | |
| DURATION | 2 WEEKS | 2 DAYS | ||
| INCIDENCE OF DEATH | 90-100% | |||
| AVERAGE TIME OF DEATH | 1-2 WEEKS | 2 DAYS | ||
| THERAPY | MAINTENANCE OF ELECTROLYTE BALANCE | SEDATIVES | ||
In stability operations and support operations, high levels of environmental contamination and the use of RDD can cause radiological injury to personnel at levels below that necessary to produce performance decrement and traditional casualty status. Treatment and evacuation guidelines will be in accordance with command guidance. Individual physical dosimetry is the most expedient measurement technique for this exposure (see Table A-10). These radiation injuries and effects may also be seen in war; especially, from hostile forces employment of RDDs.
Table A-10. Stability Operations and Support Operations: Radiation Injuries and Effects of Radiation Exposure of Personnel
| RADIATION EXPOSURE STATUS | TOTAL CUMULATIVE DOSE | STOCHASTIC RISK LONG-TERM HEALTH EFFECTS | MEDICAL NOTE | MEDICAL ACTIONS |
|---|---|---|---|---|
| 0 | <0.05 cGy | NORMAL RISK. | US BASELINE 20% LIFETIME RISK OF FATAL CANCER. | RECORD IN EXPOSURE RECORD— IF NORMALLY MONITORED PERSONNEL. |
| 1A | 0.05 TO 0.5 cGy | UP TO 0.04% INCREASED RISK LIFETIME FATAL CANCER. | NONE (0.001 Sv US ANNUAL GENERAL POPULATION EXPOSURE LIMIT.) | RECORD AS HISTORY IN MEDICAL RECORD—TACTICAL OPERATION EXPOSURE. |
| 1B | 0.5 TO 5 cGy | US RADIATION OCCUPATIONAL RISK. 0.04%-0.4% INCREASED RISK LIFETIME CANCER. | REASSURANCE (0.05 Sv US ANNUAL OCCUPATIONAL LIMIT.) | RECORD IN MEDICAL RECORD—TACTICAL OPERATION EXPOSURE. |
| 1C | 5 TO 10 cGy | 0.4%-0.8% INCREASED RISK LIFETIME FATAL CANCER. | COUNSEL REGARDING INCREASED LONG-TERM RISK. NO LIVE VIRUS VACCINES X 3 MONTHS. | RECORD IN MEDICAL RECORD—TACTICAL OPERATION EXPOSURE. |
| 1D | 10 TO 25 cGy | 0.8%-2% INCREASED RISK LIFETIME FATAL CANCER. | POTENTIAL FOR INCREASED MORBIDITY OF OTHER INJURIES OR INCIDENTAL DISEASE. <2% INCREASED LIFETIME RISK OF FATAL CANCER. | RECORD IN MEDICAL RECORD—TACTICAL OPERATION EXPOSURE. CONSIDER ROUTINE EVACUATION FROM THEATER IAW COMMANDER'S OPERATIONAL GUIDANCE. |
| 1E | 25 TO 75 cGy | 2%-5.6% INCREASED RISK LIFETIME FATAL CANCER. | INCREASED MORBIDITY OF OTHER INJURIES OR INCIDENTAL DISEASE. <6% INCREASED LIFETIME RISK OF FATAL CANCER. | RECORD IN MEDICAL RECORD—TACTICAL OPERATIONAL EXPOSURE. CONSIDER EXPEDITED EVACUATION FROM THEATER IAW COMMANDER'S OPERATIONAL GUIDANCE. |
Biological warfare is the intentional use, by an enemy, of live agents or toxins to cause death and disease among personnel, animals, and plants, or to deteriorate materiel.
a. Live Agents.
(1) Live agents are living organisms like viruses, bacteria, and fungi. They can be delivered directly (artillery or aircraft spray), or through a vector such as a flea or tick. Advances in modern weaponizing of biological agents have become easier.
(2) For some agents, only a few organisms are needed to cause infection. Live agents are small and light; they can be spread great distances by the wind and contaminate unfiltered or nonairtight places.
(3) Aerosolized particles of 1 to 5 micron (μ) size carrying live agents are small and light. They require time after they are ingested to multiply enough to overcome the body's defenses. This incubation period may vary from hours to days or weeks depending on the type of organism. Thus, to be effective, a live agent attack would need to be launched well in advance of a tactical assault.
(4) These agents are sensitive to environmental conditions (for example humidity and sunlight). Many bacterial agents will not survive outside the host organism (human and animals).
(5) Live agents are not detectable by any of the five physical senses; usually the first indication of a biological attack is the ill personnel. The diseases caused by live agents may be difficult to control when the aerosol attack is directed against a large population. Some diseases may be transmitted from person-to-person after the initial attack; examples include plague, smallpox, and some viral hemorrhagic fevers.
(6) Because of their incubation period and life cycle, likely areas for live agent use are in the combat service support (CSS) area; but attacks in forward areas cannot be ruled out.
b. Spore Forming Biological Agents. Spore formers such as anthrax can survive for an extended time, even under very adverse environmental conditions (dry, extremes of temperatures, and flooding). Once inhaled, ingested, or injected into the human body, the spores germinate and produce the illness.
c. Toxins.
(1) Toxins are by-products (poisons) produced by plants, animals, or microorganisms. It is the poisons that harm man, not the organisms that make the toxins. In the past, the only way to deliver toxins on a large scale was by using the organism. With today's technology large quantities of many toxins can be produced; thus, they can be delivered without the accompanying organism.
(2) Toxins have several desirable traits. They are poisonous compounds that do not grow, reproduce, or die after they have been dispersed; they are more easily controlled than live organisms. Field [A-15] monitors capable of providing prompt warning of a toxin attack are not available; therefore, personnel must learn to quickly recognize signs of attack, such as observing unexplained symptoms of victims. Toxins produce effects similar to those caused by chemical agents; however, the victims will not respond to the first-aid measures that work against chemical agents. Unlike live agents, mycotoxins (T2) can penetrate intact skin; other toxins cannot. Because the effects on the body are direct, the symptoms of an attack may appear very rapidly. The potency of most toxins is such that very small doses will cause injuries and/or death. Thus, their use by an enemy may be an alternative to chemical agents because it allows the use of fewer resources to cover the same or a larger area. Slight exposure at the edges of an attack area may produce severe symptoms or death from exposure to toxins because of their extreme toxicity. Lethal or injury downwind hazard zones for toxins may be far greater than those of CW agents.
Biological agents can be disseminated in a spectrum of physical states. They may be living microorganisms or spore forms of the organism. See Table A-11 for stability of various biological agents. They may be spread by—
The only requirement is that they must be stable enough to survive transport and dissemination. The toxicity of biological agents is not the same for everyone; each individual does not react exactly the same way to the same amount of an agent. Some are more resistive than others because of race, sex, age, or other factors. The dose is the quantity of a biological agent received by the subject. The penetration of agents by various routes need not be accompanied by irritation or damage to the absorbent surface. There are often unique signs and identifying symptoms depending on entry route (inhalation, ingestion, or dermal).
a. Biological agents dispersed by spray often enter the body through the respiratory tract (inhalation injury). The agent may be absorbed by any part of the respiratory tract from the mucosa of the nose and mouth to the alveoli of the lungs.
b. Liquid droplets and (less commonly) solids may be absorbed from the surface of the skin, digestive tract, and mucous membranes. Agents penetrating the skin may form temporary reservoirs under the skin.
c. Contaminated food and water can produce casualties when ingested.
Table A-11. Types and Characteristics of Some Biological Agents
| ENTRANCE | ||||
|---|---|---|---|---|
| TYPE OF AGENT | STABILITY | INCUBATION TIME | AEROSOL | NONAEROSOL |
| ANTHRAX | HIGH | HOURS TO 7 DAYS | INHALATION | SKIN, MOUTH |
| BOTULINUM TOXIN | HIGH | 24 TO 36 HOURS | INHALATION | MOUTH, WOUND |
| BRUCELLOSIS | HIGH IN WET ENVIRONMENT | 1 TO 4 WEEKS | INHALATION | MOUTH, SKIN, EYES |
| CHOLERA | MODERATE | HOURS TO 5 DAYS | MOUTH | |
| PLAGUE (PNEUMONIC) | LOW | 2 TO 4 DAYS | INHALATION | |
| PLAGUE (BUBONIC) | MODERATE | 2 TO 10 DAYS | BITE OF VECTOR | |
| RICIN | HIGH | <36 HOURS | INHALATION | MOUTH |
| SMALLPOX | HIGH | 7 TO 17 DAYS | INHALATION | LESION CONTACT |
| STAPHYLOCOCCAL ENTEROTOXIN B | HIGH | 1 TO 6 HOURS | INHALATION | MOUTH |
| TRICHOTHECENE MYCOTOXIN | HIGH | MINUTES TO HOURS | INHALATION | MOUTH, SKIN |
| TULAREMIA | LOW | 2 TO 10 DAYS | INHALATION | MOUTH, SKIN, BITE OF VECTOR |
| VENEZUELAN EQUINE ENCEPHALITIS | MODERATE | 1 TO 6 DAYS | INHALATION | BITE OF VECTORS |
| VIRAL HEMORRHAGIC FEVERS | LOW | DAYS TO MONTHS | INHALATION | BITE OF VECTORS |
a. Management. Management of patients suffering from the effects of BW agents may include the need for isolation. Barrier nursing for patients suspected of suffering from exposure to BW agents will reduce the possibility of spreading the disease to health care providers and other patients. Specimens must be collected and submitted to the designated supporting laboratory for identification. For details on hospital infection control aspects of managing BW casualties, see FM 8-284.
b. Mass Casualty. A BW agent attack can produce a mass casualty situation at all levels of HSS. A major problem with a BW mass casualty situation is that HSS personnel are more susceptible to becoming a casualty to BW agents. Also, the ill patient may be the first indicator that a BW agent has been dispersed.
c. Decontamination. Decontamination is an individual and unit responsibility. However, some individuals may arrive at the MTF that have not been decontaminated or that become contaminated en route [A-17] to the MTF. These individuals must be decontaminated at the MTF before they are admitted to prevent contamination of the MTF and exposure of medical personnel to the biological agent. See Appendix G for details on patient decontamination.
d. Treatment. Specific treatment is dependent upon the BW agent used. Patients are treated for symptomatic presentation unless the BW agent identity is known. Field Manuals 8-9 and 8-284 provide detailed information on medical management and treatment.
a. A chemical agent is a chemical that is used to kill, seriously injure, or incapacitate man because of its physiological effects. They can be disseminated by artillery, aircraft, rocket, or by nonconventional means used by terrorists. When first employed in combat during World War I, the chemical weapon (chlorine) was so effective that the attacking Germans were not prepared to exploit the success.
b. Chemical agents are very effective weapons against poorly trained and equipped forces; however, they are less effective against well-trained forces.
Chemical agents can be disseminated as a gas, vapor, or aerosol under ambient conditions. They have a range of odors varying from none to highly pungent characteristics. Their stability is dependent upon the environmental conditions in the area of employment. See Table A-11 for persistency of various chemical agents.
a. The toxicity of a chemical agent is not the same for everyone; each individual does not react exactly the same way to the same amount of an agent. Some are more resistant than others because of physiological factors. The dose is the quantity of a chemical received by the individual for percutaneous or oral doses and as a time-weighted concentration, milligrams-minute (m3), for inhalation. It is usually expressed as milligrams of agent per kilogram of subject body weight (mg/kg). The LD50 is the dose that kills 50 percent of the exposed population. The incapacitation dose 50 (ID50) is the incapacitation dose for 50 percent of the exposed subjects. The penetration of agents by various routes need not be accompanied by irritation or delayed superficial damage to the absorbent surface, but there are often unique signs and symptoms identifiable by the route of entry.
(1) Gaseous, vapor, and aerosol chemical agents often enter the body through the respiratory tract (inhalation injury). The agent may be absorbed by any part of the respiratory tract from the mucosa of the nose and mouth to the alveoli of the lungs. Aerosol particles larger than 5 μ tend to be retained in the upper respiratory tract; particles in the 1 to 5 μ range are retained in the deep volume of the lungs; while those below 1 μ tend to be breathed in and out again; although a few are retained in the deep volume of the lungs.
(2) Vapors and droplets of liquids can be absorbed from the surface of the skin and mucous membranes. Toxic compounds that are harmful to the skin can produce their effects in liquid or solid state. Agents penetrating the skin may form temporary reservoirs under the skin; the vapors of some volatile [A-18] liquids can penetrate the skin and cause intoxication. Additionally, wounds and abrasions may present areas that are more permeable than intact skin.
b. Chemical agents may be divided into two main categories (persistent and nonpersistent) that describe how long they are capable of producing casualties. Table A-12 lists the common chemical agents, their effects and time of effectiveness. Table A-13 lists the types and characteristics of common chemical agents.
(1) Persistent agents continue to present a hazard for considerable periods (days) after delivery by remaining as a contact hazard, or by slowly vaporizing to produce a hazard by inhalation.
(2) Nonpersistent agents disperse rapidly after release and present an immediate, short duration (hours) hazard. They are released as airborne particles, aerosols, and gases.
Table A-12. Common Chemical Warfare Agents
| COMMON NAME | EFFECT | TIME TO EFFECT |
|---|---|---|
| TABUN (GA) | LETHAL NERVE AGENTS | INHALATION: SECONDS TO MINUTES TOPICAL: MINUTES INGESTION: MINUTES TO HOURS |
| SARIN (GB) | ||
| SOMAN (GD) | ||
| V-AGENTS | ||
| HYDROGEN CYANIDE | LETHAL BLOOD AGENT | MINUTES |
| MUSTARD | BLISTER AGENTS | 1 TO 12 MINUTES |
| LEWISITE | MINUTES | |
| LSD AND BZ | INCAPACITATING AGENTS | 15 TO 60 MINUTES |
| PHOSGENE | LUNG-DAMAGING (CHOKING) | MINUTES |
| CHLORINE | SECONDS TO MINUTES | |
Table A-13. Types and Characteristics of Chemical Agents
| TYPE OF AGENT | SYMBOL | PERSISTENCE | RATE OF ACTION | ENTRANCE | ||
|---|---|---|---|---|---|---|
| SUMMER | WINTER | VAPOR/AEROSOL | LIQUID | |||
| NERVE | GA, GB, GD | 10 MIN-24 HR | 2 HR-3 DAYS | VERY QUICK | EYES, LUNGS | EYES, SKIN, MOUTH |
| VX | 2 DAYS-1 WK | 2 DAYS-WEEKS | QUICK | EYES, LUNGS | EYES, SKIN, MOUTH | |
| CHOKING | CG, DP | 1-10 MIN | 10 MIN-1 HR | IMMEDIATE | LUNGS | EYES |
| BLISTER | HD, HN | 3 DAYS-1 WK | WEEKS | SLOW | EYES, SKIN, LUNGS | EYES, SKIN |
| L, HL | 1-3 DAYS | WEEKS | QUICK | EYES, SKIN, LUNGS | EYES, SKIN, MOUTH | |
| CX | DAYS | DAYS | VERY QUICK | EYES, LUNGS, SKIN | EYES, SKIN, MOUTH | |
| BLOOD | AC, CK | 1-10 MIN | 10 MIN-1 HR | VERY QUICK | EYES, LUNGS | EYES, MOUTH, INJURED SKIN |
The effectiveness of a chemical agent is a measure of how much agent is required to produce the desired effect. Thus, an agent that is toxic at a lower dose than another similar agent is more effective. Besides dose required for a given effect, persistency may be used to measure effectiveness. Persistency depends on the agent's physical characteristics, the amount of agent delivered, its physical state, weapons system used, the terrain, and weather in the target area. The desired effects will determine the physical, chemical, and toxicological properties of the chemical agent employed.
a. Nerve agents are primarily organophosphorus esters similar to insecticides. Those of military importance are combined under this term. Although some have been given names, they are usually known by their code letters: GA; GB; GD; and VX. They are all liquids, varying in volatility that is in a range between gasoline and heavy lubricating oil. Their freezing points are -40 degrees Celsius or lower.
(1) Liquid nerve agents are pale yellow to colorless and are almost odorless. They are moderately soluble in water and highly soluble in lipids (oil). They are rapidly destroyed by strong alkalies and chlorinating compounds. Normal clothing is readily penetrated by liquid or vapor agents. Butyl rubber and synthetic material are more resistant than natural fibers. Agents can penetrate into nonabsorbent material such as web belts and can continue to present a hazard by desorption (off-gassing) of the vapor. Although local sweating and twitching may occur, usually there is no local irritant change after cutaneous exposure. Toxicity depends upon the route of entry and physical characteristics.
(2) Nerve agents strongly inhibit the cholinesterase enzymes. When acetylcholine is released by the nerve junction, it is hydrolyzed by the enzyme. Acetylcholine is the chemical mediator for transmission of the nerve impulses in numerous synapses of the central nervous system (CNS) and the autonomic nervous system and at the endings of the cholinergic nerves (for example: affecting the smooth muscles of the iris, ciliary, bronchial tree, and gastrointestinal tract). The inhibition of cholinesterase by nerve agents is almost irreversible, so the effects are prolonged. Until the cholinesterase level is restored to normal, there is an increased susceptibility to nerve agent exposure. During this time, the effects of [A-20] repeated exposure are cumulative and the patient may feel "subpar" (for example: tired, fatigue easily, poor appetite, impaired concentration) until recovery is complete.
(3) Nerve agent poisoning is easily identified by the characteristic signs and symptoms as follows:
(a) MILD symptoms (self-aid). Casualties with MILD symptoms may experience most or all of the following:
(b) Casualties with MODERATE symptoms (buddy aid) will experience an increase in the severity of most or all of the MILD symptoms. Especially prominent will be an increase in fatigue, weakness, and muscle fasciculations. The progress of symptoms from MILD to MODERATE indicates either inadequate atropine treatment or continuing exposure to agent.
(c) SEVERE symptoms (buddy aid). Casualties with SEVERE symptoms may experience most or all of the MILD symptoms, plus most or all of the following:
b. There are three major families of blister agents (vesicants); HD and HN, L, and CX. Most vesicants (except CX) are relatively persistent. Mustards can modify the structure of nucleic acids, cellular membranes, and proteins by combining with certain functional groups (particularly the sulfhydryl-containing enzymes) for which they have an affinity.
(1) The cutaneous syndrome is divided into four phases: latent, erythema, vesication, and necrosis. Vesicants can penetrate the skin by contact with either liquid or vapor. The latent period is characteristic of the agent. For mustards it is usually several hours, for L it is short, and for CX it is negligible. The latent period is also affected by the dose, temperature, and humidity. The symptoms of the erythema phase are red, painful itching followed by painful necrosis that heals slowly.
(2) In the eyes, vesicants produce intense pain and photophobia. Blistering of the eyelids and mucous membranes can result in temporary blindness. Even after recovery, scars on the cornea can reduce visual acuity.
(3) In the respiratory tract, these agents attack the mucous membranes irritating them. They can paralyze vocal chords and can lead to chemical pneumonitis, or possibly death.
(4) Although blister agents can affect other organs and produce deleterious effects, the skin, eyes, and respiratory tract are the principle organs effected.
c. Chemical agents that attack lung tissue (choking agents) and cause pulmonary edema are classed as lung damaging agents. Choking agents consist of CG and DP, CL, and PS. Phosgene is typical of the lung-damaging agents; it is used as the example here.
(1) Phosgene is a colorless gas that has an odor resembling new mown hay. Although effects are primarily confined to the lungs, phosgene may also cause mild irritation of the eyes and upper respiratory tract. Phosgene causes a shift in the membrane potential of the alveoli allowing the passage of fluid into the alveoli, resulting in massive pulmonary edema and severely impairing the exchange of oxygen (O2) and carbon dioxide (CO2) between the capillary blood and the alveolar air.
(2) Initially hypoxemia occurs and is followed shortly by hyperventilation when the frothy edema fluid fills the bronchioli and CO2 expiration stops.
(3) Signs and symptoms during and immediately following exposure are coughing, tightness of chest, nausea, occasionally vomiting, headache, and lacrimation (tearing).
d. Blood agents consist of AC and CK; both are readily absorbed by the mucous membranes and the intact skin. The odor of AC resembles bitter almonds, but many people cannot detect it. Detecting the [A-22] odor of CK is difficult because of its irritating and lacrimatory effects. It is also poorly absorbed by the metallic salt-impregnated charcoal filters in the protective mask. These agents inhibit certain enzymes (particularly cytochrome oxidase) that are important for oxidation-reduction in the cells; therefore, cell respiration is inhibited and oxygen carried by the hemoglobin is not consumed causing the venous blood to remain bright red. Initial symptoms are characterized by violent convulsions, increased deep respiratory movements, followed by cessation of respiration within one minute, slowing of heart rate to death. High concentrations exert their effects rapidly; however, if the patient is still alive after the cloud has passed, he will probably recover spontaneously.
e. Incapacitating agents are chemicals that produce a temporary disabling condition that persists for hours to days after exposure to the agent has ceased (unlike that produced by riot control agents). While not required, medical treatment produces a more rapid recovery. Characteristics of these agents are that they—
The two types likely to be encountered are CNS depressants and CNS stimulants.
(1) Central nervous system depressants are compounds that have a predominant effect of depressing or blocking the activity of the CNS; often by interfering with the transmission of information across synapses. An example of this type of agent is BZ. The action of acetylcholine, both peripherally and centrally, appears to be blocked by BZ. Low doses disrupt higher integrative functions of memory, problem solving, attention, and comprehension. High doses produce toxic delirium that destroys the ability to perform any military task. Within the CNS, BZ seems to produce its effects in the same way as atropine. Small doses cause sleepiness and decreased alertness with elevated heart rate, dry skin and eyelids, drowsiness, increased pupil size, and elevated skin temperatures. Progressive intoxication is marked by an inability to respond effectively to the environment (4 to 12 hours), followed by increasing activity and random/unpredictable behavior (12 to 96 hours). Because the patient cannot sweat, heat stress becomes a problem.
(2) Central nervous system stimulants are agents that cause excessive nervous activity, often by boosting or facilitating transmission of impulses across synapses. The effect is to "flood" the cortex and other higher regulatory centers with too much information, making concentration difficult and causing indecisiveness and an inability to act. These include LSD, psilocybin, and mescaline. Intoxication shows sympathetic stimulation (rapid heart rate, sweaty palms, pupillar enlargement, and cold extremities) and mental excitation (nervousness, trembling, anxiety, and inability to relax or sleep); feelings of tension, exhilaration, heightened awareness, paranoid ideas, and profound states of terror may also occur.
a. Management. Movement of chemical agent casualties can spread the contamination to clean areas. All casualties are decontaminated as far forward as the situation permits. All patients must be decontaminated before they are admitted into a clean MTF. The admission of one contaminated patient into an MTF will contaminate the facility; thereby reducing its treatment capabilities.
b. Mass Casualty. A mass casualty situation is presented when chemical agents are employed. Additional HSS personnel and equipment must be provided in a short period of time if the level of care is to be maintained. Treatment at far forward MTFs is limited to life- or limb-saving care. Patients that can survive evacuation to the next level of care are not treated at the forward facility. This provides time for treating those patients that cannot survive the evacuation time.
c. Decontamination. Decontamination is an individual and unit responsibility. However, some individuals may arrive at the MTF that have not been decontaminated or that become contaminated en route to the MTF. These individuals must be decontaminated at the MTF before they are admitted to prevent contamination of the MTF and exposure of medical personnel to the chemical. See Appendix G for detailed information on patient decontamination procedures.
d. Treatment. Field Manuals 8-9 and 8-285 provide treatment procedures for chemical agent patients.
a. Management. Movement of TIM casualties can spread the contamination to clean areas. All casualties are decontaminated as close to the incident site as possible. All patients must be decontaminated before they are admitted into a clean MTF. The admission of one contaminated patient into an MTF may contaminate the facility; thereby reducing its treatment capabilities.
b. Mass Casualty. A mass casualty situation is presented when the number of casualties exceeds the capabilities of medical personnel at the location to provide needed care at the incident site. Treatment at the incident site is limited to life- or limb-saving care. Patients that can survive are evacuated to the nearest MTF with a patient decontamination capability.
c. Decontamination. Decontamination is an individual and first responder responsibility. However, some individuals that self evacuated or were evacuated due to the mass casualty situation arrive at the MTF that have not been decontaminated. These individuals must be decontaminated at the MTF before they are admitted to prevent contamination of the MTF and exposure of unprotected medical personnel and other patients to the TIM. See FM 8-500 for detailed information on decontamination procedures for TIM contaminated casualties.
d. Treatment. Field Manual 8-500 provides treatment procedures for some TIM casualties. Treatment for many TIM casualties is agent specific and receiving MTFs must be prepared for these events.
EXAMPLE: Treatment for a casualty exposed to toxic levels of an inorganic phosphate pesticide would be treated in the same manner as a nerve agent casualty except the amount of antidote for the pesticide poisoned casualty will be many times greater than for the nerve agent casualty.
a. Critical elements for accuracy in analysis of NBC samples and physiological specimens are correct collecting, packaging, handling, and transporting techniques. The quality of any analytical evaluation is directly related to the quality of the sample/specimen and the degree of postcollection degradation that occurs prior to testing. Health service support personnel collect and submit specimens for suspect NBC hazards/agents involving humans and animals. Chemical corps and other nonmedical units collect and submit environmental (air, plant, and soil) samples for suspect NBC hazards/agents. Preventive medicine personnel collect and submit water and ice samples for suspect NBC hazards/agents. Veterinary personnel collect and submit food samples, such as fruits and vegetables, and specimens from animals for suspect NBC hazards/agents. Specimens collected from patients that are suspect of being exposed to a biological agent are forwarded to the supporting medical laboratory (such as the TAML, AML or US Navy Forward Deployed PVNTMED Unit) for analysis.
b. Essentially all military operations from war to stability operations and support operations may generate medical laboratory testing requirements. Each scenario, geographical region, population base, and suspect agent will impact on the type and amount of samples/specimens required and the collection process. During all operations, express permission is required before collecting specimens from civilians because of religious or sociological beliefs in many cultures. To obtain such specimens without permission could result in unnecessary mission complications.
NOTES
1. The term "sample" refers to nonhuman and nonanimal origin.
The term "specimen" refers to human and animal origin.
2. Always consider that chemical agents may have been employed.
Check for chemical agents before collecting a biological sample/specimen.
Chemical agents can damage or destroy biological agents.
Also, chemical agents not identified in the sample/specimen can pose
a hazard to receiving laboratory personnel. Mark all samples that are
potentially contaminated with chemical agents as such.
3. Precautions should be taken to protect the sample/specimen
collector from potential BW agents; at a minimum, respiratory
protection and rubber gloves must be worn. Additional care must
be taken when collecting samples/specimens to prevent cross-contamination.
Gloves must be changed or decontaminated between
sample/specimen collections.
[B-2]
4. Samples will not be delivered to the clinical laboratory of an
MTF for analysis. They must be delivered to the designated supporting
medical laboratory for processing. This will prevent accidentally
spreading a biological agent in the MTF.
c. Coordination for follow-on testing is absolutely critical to the sample/specimen collection process.
d. Coordination with the receiving laboratory should be made to establish sample requirements, preferred collection techniques, methods of preservation, and transportation conditions, when the tactical situation and/or mission permits.
e. The number of medical specimens that need to be collected varies with the type of analysis performed and the impact of the values determined. The number and types of "control" samples/specimens required to validate test information is determined by the supporting medical laboratory personnel. Random sampling, matched with control populations, or other techniques will be employed as the requirements are identified.
a. A complete history of the circumstances about each sample's/specimen's acquisition must be provided to the agency conducting the analysis.
b. Critical information includes, but is not limited to—
a. Ante mortem Specimens. Physiological specimens from living human or animal patients can include just about any conceivable body source or excreted by-product. It must be noted that specimen types are seldom interchangeable; the exact type and amount of specimen required for a specific assay must be known before a collection procedure is initiated (see Table B-1).
NOTE
In cases where the supporting laboratory cannot be contacted, as a minimum the following specimens should be collected: Urine—25 to 50 ml in a sterile container. Blood—two 7 to 10 ml tubes without anticoagulant (red-stopper VacutainerTM); two 7 to 10 ml tubes with potassium or sodium ethylenediaminetetraacetate (EDTA) (lavender-stopper VacutainerTM).
b. Post mortem and Forensic Specimens. The analysis of specimens from deceased humans and animals can provide valuable information about the disease, organisms, injuries, or environmental conditions at the time of death. This information can greatly enhance the treatment of others affected by the same, or physiologically similar, process. Specimen collection for post mortem or forensic examination is very important; the techniques involved reflect a significant degree of training, experience, and skill. Most specimens will be of the same type and size as for ante mortem specimens, but types and amounts of specimens will be determined by the collector.
(1) The collection of specimens from remains should be conducted exclusively by a pathologist, or other personnel specifically trained in forensic collection techniques. An exception is when Special Operations Forces (SOF) personnel are operating under radio silence conditions; the most qualified medical person with the operation collects, preserves, and transports or coordinates transport of specimens for evaluation. The same chain of custody requirements applies to specimens collected by SOF personnel, as with all other specimens.
(2) A large amount of support information can be gained by analyzing the site of injury and subsequent death. This "site scene" investigation requires a tremendous attention to detail and a trained observer. If forensic personnel cannot be contacted, or will be unduly delayed in arriving at the scene, then photographs of the victim and the immediate surroundings should be made. The scope and extent of the photographs should be composed to reflect as much detail as possible to assist forensic personnel in reviewing the scene retrospectively. In the event that photography is not feasible, detailed sketches of the scene should be made to assist the forensic investigation.
(3) Techniques such as cardiac or bladder puncture, needle biopsy of organs, spinal tap, or exploratory laparotomy will not be performed by untrained personnel unless specifically requested and directed by forensic investigators.
Table B-1. Specimen Collection for Suspect Biological Warfare Agents
| EARLY POSTEXPOSURE | CLINICAL | CONVALESCENT/TERMINAL/POSTMORTEM |
|---|---|---|
| ANTHRAX | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | 3 TO 10 DAYS. |
| NASAL AND THROAT SWABS, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE, FA, AND PCR. | SERUM (TT OR RT) FOR TOXIN ASSAYS. BLOOD (E, C, H) FOR PCR. BLOOD (BC OR C) FOR CULTURES. |
SERUM (TT OR RT) FOR TOXIN ASSAYS. BLOOD (BC OR C) FOR CULTURE. PATHOLOGY SPECIMENS. |
| PLAGUE | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | >6 DAYS. |
| NASAL SWABS, SPUTUM, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE, FA, AND PCR. | BLOOD (BC AND C) FOR CULTURE AND BLOODY SPUTUM (C) FOR FA. SERUM (TT OR RT) FOR F-1 ANTIGEN ASSAYS. BLOOD (E, C, OR H) FOR PCR. |
SERUM (TT OR RT) FOR IgM, LATER FOR IgG. PATHOLOGY SPECIMENS. |
| TULAREMIA | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | >6 DAYS. |
| NASAL SWABS, SPUTUM, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE, FA, AND PCR. | BLOOD (BC OR C) FOR CULTURE. BLOOD (E, C, OR H) FOR PCR. SPUTUM FOR FA AND PCR. |
SERUM (TT OR RT) FOR IgM AND LATER IgG, AGGLUTINATION TITERS. PATHOLOGY SPECIMENS. |
| MELIOIDOSIS/GLANDERS | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | >6 DAYS. |
| NASAL SWABS, SPUTUM, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE AND PCR. | BLOOD (BC OR C) FOR CULTURE. BLOOD (E, C, OR H) FOR PCR. SPUTUM AND DRAINAGE FROM SKIN LESIONS FOR PCR AND CULTURE. |
BLOOD (BC OR C) AND TISSUE FOR CULTURE. SERUM (TT OR RT) FOR IMMUNOASSAYS. PATHOLOGY SPECIMENS. |
| BRUCELLOSIS | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | >6 DAYS. |
| NASAL SWABS, SPUTUM, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE AND PCR. | BLOOD (BC OR C) FOR CULTURE. BLOOD (E, C, AND H) FOR PCR. |
BLOOD (BC OR C) AND TISSUE FOR CULTURE. SERUM (TT OR RT) FOR IMMUNOASSAYS. PATHOLOGY SPECIMENS. |
| Q FEVER | ||
| 0 TO 24 HOURS. | 2 TO 5 DAYS. | >6 DAYS. |
| NASAL SWABS, SPUTUM, AND INDUCED RESPIRATORY SECRETIONS FOR CULTURE AND PCR. | BLOOD (BC OR C) FOR CULTURE IN EGGS OR MOUSE INOCULATION. BLOOD (E, C, AND H) FOR PCR. |
BLOOD (BC OR C) FOR CULTURE IN EGGS OR MOUSE INOCULATION. PATHOLOGY SPECIMENS. |
| BOTULISM | ||
| 0 TO 24 HOURS. | 24 TO 72 HOURS. | >6 DAYS. |
| NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR PCR (CONTAMINATING BACTERIAL DNA) AND TOXIN ASSAYS. SERUM (TT OR RT) FOR TOXIN ASSAYS. |
NASAL SWABS AND RESPIRATORY SECRETIONS FOR PCR (CONTAMINATING BACTERIAL DNA) AND TOXIN ASSAYS. | USUALLY NO IgM OR IgG. PATHOLOGY SPECIMENS (LIVER AND SPLEEN FOR TOXIN DETECTION). |
| [B-7]RICIN INTOXICATION | ||
| 0 TO 24 HOURS. | 36 TO 48 HOURS. | >6 DAYS. |
| NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR PCR (CONTAMINATING CASTOR BEAN DNA) AND TOXIN ASSAYS. SERUM (TT OR RT) FOR TOXIN ASSAYS. |
SERUM (TT OR RT) FOR TOXIN ASSAY. TISSUE FOR IMMUNOHISTOLOGICAL STAINING. PATHOLOGY SPECIMENS. | SERUM (TT OR RT) FOR IgM AND IgG IN SURVIVORS. |
| STAPH ENTEROTOXICOSIS | ||
| 0 TO 3 HOURS. | 2 TO 6 HOURS. | >6 DAYS. |
| NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR PCR (CONTAMINATING BACTERIAL DNA) AND TOXIN ASSAYS. SERUM (TT OR RT) FOR TOXIN ASSAYS. |
URINE FOR IMMUNOASSAYS. NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR PCR (CONTAMINATING BACTERIAL DNA) AND TOXIN ASSAYS. SERUM (TT OR RT) FOR TOXIN ASSAYS. |
SERUM FOR IgM AND IgG. |
| T-2 TOXICOSIS | ||
| 0 TO 24 HOURS POSTEXPOSURE | 1 TO 5 DAYS. | >6 DAYS POSTEXPOSURE. |
| NASAL AND THROAT SWABS AND INDUCED RESPIRATORY SECRETIONS FOR IMMUNOASSAYS, HPLC/MASS SPECTROMETRY. | SERUM (TT OR RT) AND TISSUE FOR TOXIN DETECTION. | URINE FOR DETECTION OF TOXIN METABOLITES. |
| EQUINE ENCEPHALOMYELITIS | ||
| (VEE, EEE, AND WEE VIRUSES) | 24 TO 72 HOURS. | >6 DAYS. |
| 0 TO 24 HOURS. NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR RT-PCR AND VIRAL CULTURE. |
SERUM (TT OR RT) AND THROAT FOR CULTURE. SERUM (E, C, H, TT, OR RT) FOR RT-PCR. THROAT SWABS UP TO 5 DAYS FOR CULTURE THEN CSF. SERUM (TT OR RT) FOR ANTIGEN ELISA. |
SERUM (TT OR RT) FOR IgM. PATHOLOGY SPECIMENS PLUS BRAIN. |
| POX) | ||
| (SMALLPOX AND MONKEYPOX) | 2 TO 5 DAYS. | >6 DAYS. |
| 0 TO 24 HOURS. NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR PCR AND VIRAL CULTURE. |
SERUM (TT OR RT) FOR VIRAL CULTURE. | SERUM (TT OR RT) FOR VIRAL CULTURE. DRAINAGE FROM SKIN LESIONS/ SCRAPINGS FOR MICROSCOPY, EM, VIRAL CULTURE, AND PCR. PATHOLOGY SPECIMENS. |
| EBOLA | ||
| 0 TO 24 HOURS. | 2 TO 5 DAYS. | >6 DAYS. |
| NASAL SWABS AND INDUCED RESPIRATORY SECRETIONS FOR RT-PCR AND VIRAL CULTURE. | SERUM (TT OR RT) FOR VIRAL CULTURE. | SERUM (TT OR RT) FOR VIRAL CULTURE. PATHOLOGY SPECIMENS PLUS ADRENAL GLAND. |
| LEGEND: | ||
| BC | Blood culture |
| C | Citrated blood |
| CSF | cerebrospinal fluid |
| DNA | deoxyribonucleic acid |
| E | EDTA |
| EEE | eastern equine encephalitis |
| ELISA | enzyme-linked immunosorbent assay |
| EM | electron microscopy |
| F-1 | fraction-1 |
| FA | fluorescent antibody |
| H | Heparin |
| HPLC | high-pressure liquid chromatography |
| IgG | immunoglobulin class G |
| IgM | immunoglobulin class M |
| PCR | polymerase chain reaction |
| RT | Red Top, if TT is not available |
| RT-PCR | reverse transcriptase/ polymerase chain reaction |
| TT | Tiger top |
| VEE | Venezuelan equine encephalitis |
| WEE | western equine encephalitis |
c. Water Sample Collection.
(1) Water samples for identification or verification of biological agent contamination are collected by PVNTMED personnel. The supporting laboratory should provide guidance on sampling procedures and collecting kits for use in collecting the samples. In the absence of guidance, a technique for use of the Sep-PakTM is described in FM 3-19.
(2) When sampling kits are not available, samples may be collected in other available sterile containers. The best containers for use are the 100-ml glass bottles used for collecting routine water samples. All water samples must be collected and placed in a cooler or refrigerator until the sample is transported to its destination. During transportation the samples must be maintained at a temperature between 1°C and 4°C.
d. Food Samples. Veterinary personnel must collect suspect biologically contaminated food samples for submission to the supporting laboratory for in-theater verification of contamination. All food samples must be collected and placed in sterile containers. Place the samples in a cooler or refrigerator until the sample is transported to its destination. During transportation the samples must be maintained at a temperature between 1°C and 4°C.
e. Animal Specimens. Veterinary personnel collect specimens from suspect biologically contaminated/diseased animals. The same types and amounts of specimens are prepared and shipped in the same manner as are human specimens.
f. Environmental Samples. Environmental samples are collected as directed in the operators' manual or other publications for operating collection systems. Example: The Biological Integrated Detection System (BIDS) collects an environmental sample using a single liquid sample collector. The collector is a high-volume aerosol sampling and collection device. On demand it samples ambient air through a two-stage virtual impactor that concentrates aerosol particles in the 2 to 10 micrometer diameter-size range. The concentrate particle stream is directed through a wet collector containing a buffer solution and, over a 45-minute period, a 40 to 50 ml sample is collected. On order or when test results indicate a suspected agent, the sample and associated documentation are packaged and transported IAW FM 3-101-4.
a. A strict chain of custody must be maintained for every sample/specimen collected. Use DD Form 1911 (Material Courier Receipt), or other document (such as DA Form 4137 [Evidence/Property Custody Document]) as directed for each sample/specimen collected. The chain of custody document must accompany the sample/specimen during transport from the point of collection to the final receiving laboratory. Each time the sample/specimen is transferred to another individual, the receiving person must sign the document to show that they received the sample/specimen and state what happened to the sample/specimen while in their custody. The document will provide the answer to the following questions:
b. The samples/specimens must be appropriately packaged, labeled, and evacuated to the designated medical laboratory for confirmation of a biological attack. The standard chain of custody for the evacuation would be as follows:
The collection of environmental, and background (control) samples/medical specimens is an integral part of investigating allegations pertaining to the first use of chemical or biological agents. The types of samples/specimens taken and the collection methods primarily depend upon the circumstances encountered by the collector. During all chemical and biological sampling operations, the commander establishes the required protective equipment to fit the situation. This appendix includes a recommended list of equipment for use during chemical and biological sampling operations (Table B-2).
Table B-2. Example NBC Collection and Shipping Equipment List
| AMOUNT | DESCRIPTION | STOCK NUMBER | |
|---|---|---|---|
| 20 | LABELS, PAPER, PRESSURE SENSITIVE | 7530-00-577-4376 | |
| 2 | GLOVES, 8-8½, EDMONT WILSONTM | 8415-00-JO2-2902 | |
| 2 | GLOVES, 9-9½, EDMONT WILSONTM | 8415-00-634-4639 | |
| 1 | TAPE, ADHESIVE, PRESSURE SENSITIVE, 2 INCH | 7510-00-159-4450 | |
| 1 | PLIERS, #47, 5 INCHES | 6520-00-543-5330 | |
| 1 | SCREWDRIVER, FLAT TIP, ¼ INCH | 5120-00-596-865 | |
| 1 | TONGS, TEFLONTM TIPS | AF 15-202-5 | |
| 2 | MICROSPATULA, WITH TEFLONTM ENDS | AF 21-401-50A | |
| 1 | SCISSORS, UNIVERSAL TYPE | AF 08-951-30 | |
| 1 | SCOOP, POLYPROPYLENE, 5X2X2 | ASP S1021-5 | |
| 2 | SPOON/SPATULA WITH TEFLONTM | AF 14-356-10 | |
| 1 | KNIFE, POCKET | 5110-00-526-8740 | |
| 5 | BOTTLES, SAMPLE, POLYETHYLENE, 6 OUNCE | CP J-6103-50 | |
| 1 | PIPET, JUMBO, TRANSFER TYPE (500/PKG) | AF 13-711-7 | |
| 10 | PIPET, GRADUAL, TRANSFER TYPE (500/PKG) | AF 13-711-9A | |
| 10 | BAG, INSULATED, TYPE I | AF 01-814-8 | |
| 10 | BAG, INSULATED, TYPE 2[*] | AF 01-814-10 | |
| 1 | BAG, WHIRL/PAK, 6 OUNCE (500/PKG) | AF 01-812-6B | |
| 1 | STRIP, pH TESTING, NONBLEEDING, PLASTIC | SW S-65271 | |
| 1 | SEP-PAKTM C18 | W51910 (50/BOX) | |
| 2 | SYRINGE, HYPODERMIC, 50 OR 60 ml | 6515-00-168-6913 | |
| 2 | STOPCOCK, THREE-WAY | ASP S8965-2 | |
| 1 | TUBING, LABORATORY, R3602 CLEAR | AF 14-169-3B | |
| 1 | PEN, MARKING, WATERPROOF | AF13-381 (12/PKG) | |
| 2 | TUBES, TENAXTM | EC ST-023 | |
| 1 | BLADE, SURGICAL, CS2L 150S | 6515-01-009-5297 | |
| 2 | PACK, ICE | CP TR-6345-20 | |
| 6 | PAD, NONADHESIVE, 3X4, 100s | 6510-00-111-0708 | |
| 4 | PAD, COOLING, CHEMICAL, 4S | 6530-00-133-4299 | |
| 2 | PIGLETTES | SPECIAL ORDER | |
| 1 | TAPE, ANTISEIZE | 8030-00-889-3535 | |
| 1 | AIR SAMPLER, PERSONAL | LSS G4980 | |
| 1 | KIT, METRIC, POCKET BUBBLE | GL4981 | |
| 2 | METHANOL | ||
| 1 | WATER, DISTILLED (5 BOTTLE/PKG) | ||
| 1 | MATCHES, WATERPROOF | ||
| 20 | RAZOR, SURGICAL PREP | 6515-00-926-2089 | |
| 10 | WATCH, WRIST | 6645-00-066-4279 | |
| 2 | PARAFILM WITH DISPENSER | 6640-01-185-3289 | |
| 2 | FLOOR SWEEP (VERMICULITE) | 8720-01-026-9419 | |
| 100 | SEALS, TAMPER-RESISTANT | ||
| 1 | A GAS METER CAPABLE OF PROVIDING ON-STATION ANALYSIS/DETECTION CAPABILITY FOR MULTIPLE GASES TO INCLUDE INDUSTRIAL GASES. | ||
| 1 | A COMBUSTIBLE GAS INDICATOR THAT INDICATES PERCENTAGE OF OXYGEN AND EXPLOSIVITY. | ||
| 1 | A GAS METER THAT DETECTS VAPOR IN PARTS PER MILLION (PPM) AND INDICATES PRESENCE OF VAPOR AND ITS STRENGTH. | ||
| 1 | SWABS, THROAT | ||
| 2 | CAN, 6 POUND, METAL | ||
| 10 | BAG, MYLAR | ||
| 1 | CONTAINER, LEAD SHIELDING (FOR RADIATION SAMPLES) | ||
| 1 | CONTAINER, SHIPPING, IATA | ||
| 1 | CHEST, ICE | ||
| 10 | BAG, PLASTIC, RECLOSABLE | ||
| [*] WILL BE REPLACED BY MYLAR BAGS | |||
The NBC recon units collect samples under various circumstances. For example, a recon unit may collect samples in an area free of hostile forces. The Special Forces NBC Reconnaissance team may collect samples within the enemy area of operations or deep into the enemy's rear area. Samples include toxic agent munitions, chemical products, air, water, soil, and vegetation. In addition, all expended material used to collect the samples should be turned in to the laboratory with the samples. This material includes items such as expended M256A1 kits, M8 and M9 paper. These items should be recovered, packaged, and shipped with the suspected samples for analysis. Different information may be derived from each type of sample; Table B-3 compares different types of samples.
Control or background samples that are collected from clean samples must be identical to the samples collected from the areas near the attack areas as baseline data. The contaminated samples must be compared to the baseline data (control samples). This is especially true if unknown or nonstandard chemical and/or suspected biological agents were employed. The analysis center uses the control samples to compare with a contaminated one. The recon unit collects control samples of soil, water, and vegetation from areas about 500 meters upwind of an alleged attack area. Control samples generically are the same as those collected in an alleged attack area. For example, if leaves from an apple tree in an attack area were collected as a suspected contaminated sample, the recon team should collect leaves (as a control sample) from an apple tree outside of the contaminated area. If water from a pond in the attack area is collected, the recon unit should collect control samples of water from a pond (not a moving stream) in a nearby clean area. The size of an environmental control sample should be about the same as the suspected contaminated sample collected from the attack area (see Table B-4, page B-20).
Table B-3. Comparison of Sample Types
| SAMPLE STABILITY | ANALYSIS | |||
|---|---|---|---|---|
| SAMPLE TYPE | INFO VALUE | TO COLLECT | TIME REQUIRED | RELIABILITY |
| AIR | GOOD | GOOD | 20 MIN | HIGH |
| WATER | GOOD | GOOD | 5 MIN | HIGH |
| SOIL | FAIR | FAIR | 5 MIN | MODERATE |
| VEGETATION | FAIR | POOR | 10 MIN | LOW |
| TISSUE | EXCELLENT | FAIR | 30 MIN | HIGH |
| BLOOD | GOOD | FAIR | 10 MIN | HIGH |
| URINE | GOOD | FAIR | 10 MIN | HIGH |
| MUNITION FRAGMENTS | FAIR | FAIR | 10 MIN | FAIR |
| PACKING MATERIALS | FAIR | FAIR | 10 MIN | FAIR |
a. Air is a good sample matrix since it is a well-mixed medium. Air from a sample site contains a static concentration of contaminants. The concentration of contaminants depends upon the flow rate of the contaminant into the environment, the wind speed, and the physical state of the contaminant, the terrain contours, and temperature as a variable. The sample should be taken within 102 meters of a contaminated surface and at the downwind edge of a contaminated area. The method should consist of pumping a given volume of air, by hand or electric pump, through sample tubes.
b. To avoid contamination, persons conducting air sampling should not use cologne, perfume, insect repellent, medical creams, or strong soaps before taking a sample. The fragrances from these products are volatile organic compounds that may be absorbed on the filter and skew analytical results. Smoke also severely interferes with air sampling, therefore, avoid tobacco and vehicle exhaust smoke.
c. The primary method for collecting air samples is with the PAS 1000 automatic air sampler in conjunction with a TenaxTM tube for a total of three to four minutes when possible. Upon completion of sampling, place the TenaxTM tube in a 2¼-inch piglette. Seal the piglette around the cap with either pressure-sensitive or TeflonTM tape. Once sealed, place the piglette into a Mylar or reclosable bag. Fold the bag around the piglette in a circular motion, then apply another bag and fold again. Once the bag is folded around the piglette, use any type tape to secure the bag around the piglette. Place the piglette into a refrigerator or cooler until the sample is transported to its destination.
d. When chemicals are permitted into the atmosphere from a facility, the best places to obtain samples are close to the emission source where the concentration of the chemical is not diluted. The further from the original point of release, the more diluted the sample becomes from mixing with air, water, or environmental pollutants.
e. Natural and man-made terrain features such as hills, valleys, and rows of buildings, sometimes aid the collector by channeling emissions. When these features are associated with a particular facility, their downwind side is a suitable place to collect a sample because the emission remains more concentrated further from the release point.
f. For collection in a possibly contaminated location, and if the situation permits, initially use a detection kit such as the M18A2/M256AI to determine if a possible vapor hazard exists from known chemical agents. Also, use the kit when personnel are required to examine possible toxic agent munitions. In any case, collect air samples with the white-band tubes and save for identification and analysis.
g. Small air samplers also enable the collector to obtain vapor samples from alleged toxic agent munitions at a safe distance while explosive ordnance disposal (EOD) operations are performed. If EOD personnel are not on the scene, the air sampler can be activated, and the collector can stand at a safe distance while the sampler is operating.
h. Perform sampling operations as soon as possible when directed by a higher headquarters or after suspected chemical or biological contamination is encountered.
a. Water sampling is a matter of collecting enough water to get acceptable information about the contaminants. The collector should provide the analysis center with one C18 and one silica cartridge when using the Sep-PakTM technique or 100 ml in a sterile bottle when Sep-PakTM is not available.
b. General guidelines: If it is believed that the threat has used standard chemical agents during an attack, use the M272 chemical agent water test kit for initial screening and sampling.
c. When collecting water samples using the Sep-PakTM C18 cartridge, the following items are required:
d. Prior to collecting each sample, prime the Sep-PakTM system in the following manner:
e. After priming the Sep-PakTM, assemble the components in the following configuration:
f. Use the following procedures to collect samples with Sep-PakTM:
NOTE
You should take a minimum of four samples: three samples of the suspected contamination and one control sample from a nearby unaffected (none contaminated) area for reference.
g. For samples to be representative of the overall contaminated area, the collection point should be carefully selected. Collect samples from—
NOTE
If an oil film, globules of organic materials, or an unnatural appearing powder-like material is visible on the water's surface, collect a surface sample of the material. If not, collect the sample from near the bottom of the water source (stream, lake, pond, water container). The upper layers of water may have lesser amounts of contaminants, due to higher temperatures that promote decomposition. Since most chemicals of interest are more dense than water, contaminants usually sink to the bottom of the water source.
h. Collect the sample without the Sep-PakTM by immersing a capped or stoppered container to the desired depth, removing the cap or stopper, letting the container fill, and then capping the container. An alternate method for deeper water is to use a plastic, pump-operated siphon to pump water from a specific depth.
i. The best time to collect a sample of water from a location is when intelligence or local reports indicate that a process of possible interest is ongoing. In the absence of reliable reporting, this may be indicated by increased activity, higher than normal amounts of security, or increased flow from facility chimneys or water discharge pipes. In field areas where a toxic agent has been sprayed or disseminated over a land area, the best time to collect water samples is just after the start of a rainstorm when runoff is beginning. Natural surface drainage will concentrate any remnants of toxic compounds in depressions, streams, or ditches.
Soil is a suitable medium to collect as samples for toxic organic compounds. A critical point, however, is that the precise site of the agent deposition must be sampled for best results. Contamination may be recognized by discoloration or apparent deposition of material on the soil's surface. If discoloration or deposits of material are evident, only collect the discolored soil or deposited materials, if possible. Dead, malformed, and wilted foliage is an indicator of contamination. Soil samples should be collected from open areas, along the drip line tents, stationary equipment, bottom of ditches and terrain depressions.
a. Collect the soil samples by using a knife, spoon, spatula, or similar item to scrape a square of topsoil (2×5×1 centimeters) from areas that appear to have been contaminated in to a collection container. If chunks or clods of earth are collected, select those that are no larger than 10×5×1 centimeters (see Table B-4). Also, collect a control sample of soil of the same type and texture from a nearby uncontaminated area.
b. Use a glass bottle, jar, or TeflonTM jar as the container when available. When these containers are not available, Mylar bags may be used. When using a glass bottle, jar, or TeflonTM jar, seal the cap with either pressure-sensitive or TeflonTM tape, and mark for identification. When using Mylar bags, place each sample in a separate bag, push excess air out, and seal by folding the open end over two to three times [B-16] and wrapping the bag with tape. Insert the first bag into a second bag, seal, tape, and mark for identification. If possible, place the samples in a piglette.
CAUTION
Avoid direct contact with the sample to prevent exposing yourself to the suspect agent (MOPP 4 is required).
c. Collect samples as soon as possible when directed, upon detection of a suspect substance, or after the alleged incident.
As with soil samples, vegetation is also a suitable medium to collect as samples for toxic organic compounds.
a. Collect samples of vegetation that appear to be different from normal. Select leaves that have wilted or appear to have been chemically burned. Collect samples of vegetation that appear to have liquid or solid substances deposited on their surfaces (this may be noticed as a shiny or moist area).
b. Collect samples of vegetation at several locations within the suspected contaminated area. Using a cutting tool or any sharp object, cut several affected leaves and/or a handful of grass whenever possible. Do not crush the sample. Place the sample into a Mylar or reclosable bag. Squeeze excess air out of the bag and seal it. Fold the open end of the Mylar bag over two to three times, and wrap it with tape. The minimum size for a sample is three leaves or three handsful of grass. One leaf is of little value, but is better than nothing. Bark is acceptable but not preferred. Mark the bag for identification. Take a control sample of similar material from an unaffected (uncontaminated) area. Fold, seal, tape, and mark the control sample in the same manner as the actual sample.
c. When it is possible to determine a probable center of attack in an area, collect vegetation samples near the center of the area, about 100 meters upwind of the area, and in several 100-meter increments downwind of the area. If the collector can discern a contamination pattern in the area, this should be reported.
a. Just as blood and urine specimens are taken from humans who were allegedly exposed in an attack, also collect specimens from individuals who claim not to be affected by a toxic agent and are from the same group as exposed personnel. The purpose is the same as collecting environmental control specimens; that is, to determine if a toxic substance is present in the individuals' natural environment or if it has been artificially introduced.
b. Selection of humans for control sampling is somewhat more complicated than selection of environmental control samples. This is because ethnic diets, racial differences, physiological makeup, and actual living conditions of persons who are outwardly similar may introduce potentially large deviations. Each of these factors must be accurately considered before selecting subjects as controls.
c. Consideration of ethnic diets is important because of unique foods or methods of food preparation that may exist. As an example, individuals in settled areas may purchase beer that has been carefully filtered and sterilized, while individuals in a nearby unsettled area may ferment their own beer by burying home crafted jugs in the ground and extracting the product little by little over several months.
d. Racial differences can account for differences in mortality and morbidity rates in specific populations. One example of this could be the high rate of hemophilia in a population versus the rarity of the disease in another.
e. Physiological makeup is critical because of the differences in hormone balance and tissue composition in males, females, adults, and juveniles. For this reason, medical control specimens should be drawn from individuals of the same gender and approximate age as specimens from exposed personnel, if possible.
f. Differences in the actual living conditions of people also require a close look. The point here is that conditions in remote, semicivilized camps are seldom the same as those in a well-established camp that has access to modern amenities.
g. The bottom line in selecting subjects for medical control sampling is that they be as similar in all aspects as possible.
a. Trained medical technicians or physicians should collect medical specimens (human or animal); however, Special Forces NBC Reconnaissance team personnel are trained to do this procedure. Remember, the collector must have express permission (authority) to collect medical specimens from the dead, because of religious beliefs in many cultures. To obtain such specimens without permission may result in unnecessary mission complications. Ensure all personnel handling or collecting medical specimens have received proper immunizations for their own protection. They must be inoculated IAW The Surgeon General's guidance.
b. Medical specimens collected during an investigation include blood, urine, sputum, nasal swabs, and tissue specimens from living victims and blood and urine specimens from unexposed persons (background control specimens).
c. Collect blood specimens using either a standard 10 cc disposable syringe with a 1- to 1½-inch needle (20 to 22 gauge), or by using a VacutainerTM system. When using a VacutainerTM system, ensure that multiple specimen needles and "red top" vacuum tubes are used. Ten cc of blood is sufficient for analytical testing. Do not take more than 5 cc from small, malnourished children. After blood is collected, it should be transferred to a polypropylene-type container and sealed with parafilm before transporting. [B-18] All body fluids should be collected without violating antiseptic techniques. Also, prior to transporting specimens, collectors need to check specimen containers for paper labels IAW guidelines for labeling medical specimens. Collect blood specimens using the following materials equipment:
NOTE
Gloves should be worn whenever handling medical specimens. Do not freeze liquid blood and urine specimens (ideal cooling temperature is between 35° and 40°F [2° to 4°C].)
d. Collect urine specimens using either a standard urine cup or by a urine catheter and urine cup. When collecting the specimen directly into a urine cup, the person must urinate into the cup until sufficient fluid is collected (40 cc of urine is preferable, although 10 cc can support analytical testing). When the person is unable to urinate, the catheterization technique is preferable. The catheterization technique is best performed in a clinical environment. As with other body fluids collected, urine must be kept cold. Do not freeze.
NOTE
For correct procedures on catheterization refer to STP 8-91W15-SM-TG.
e. Collect tissue specimens using sterile scissors and forceps or as directed by the attending physician.
(1) When casualties have unidentified skin lesions, photographs of the lesion(s) and overall photos of the extent of the lesion(s) should be taken, using color film before biopsy. A specimen of the lesion should be obtained. This is done by surgically removing a portion of the skin with a sterile pair of scissors and forceps.
(2) Place tissue specimens in a TeflonTM container filled ¼ inch from the bottom with a preservative, (formalin 10%) for preservation of the specimen until it reaches its proper destination. Seal the container and lid with parafilm. As with any other medical specimens, tissue specimens are refrigerated prior to shipment; but do not freeze tissue specimens.
f. Collect nasal swabs by using a cotton-tipped swab. Place the swab with collected specimen in a TeflonTM container filled ¼ inch from the bottom with a preservative for preservation of the specimen until it reaches its destination. Seal the container and lid with parafilm. Refrigerate the specimen for shipment, but do not freeze.
g. Collect sputum by having the patient discharge the sputum into a small, sterile screw-top jar or urine specimen cup. Seal the container and refrigerate the specimen for shipment, but do not freeze.
Post mortem specimens should be collected by individuals trained in forensics. When forensics-trained individuals are not available, the most qualified medical person should collect human specimens. Specimens from animals should be collected by veterinary personnel. In either case, the following specimens are collected:
NOTE
Before attempting any of the above procedures, collector must be certified by a qualified person (medical doctor) on the correct procedures to collect specimens from cadavers.
Table B-4. Standard Sizes of CB Samples/Specimens to be Collected
| TYPE | SIZE | NOTES |
|---|---|---|
| CHEMICAL WARFARE SAMPLES | ||
| SOIL | (10 CM X 5 CM X 1 CM) | CIGARETTE-PACK SIZE OR LARGER AREA IS MORE USEFUL THAN GREATER DEPTH |
| DILUTE AGENT | 10 ML | |
| WATER | 500 ML (MAXIMUM) | |
| C18 SEP-PAKTM | 200 ML | |
| VEGETATION | (EQUIVALENT TO 3 LEAVES OR 3 HANDSFUL OF GRASS) | DEPENDS ON AMOUNT OF CONTAMINATION. BEST SAMPLES WILL BE FOUND NEAR THE RELEASE POINT |
| BIOLOGICAL WARFARE SAMPLES | ||
| SOIL | (10 CM X 5 CM X 1 CM) | CIGARETTE-PACK SIZE OR LARGER AREA IS MORE USEFUL THAN GREATER DEPTH |
| LIQUID | 25 TO 50 ML | DO NOT USE C18 SEP-PAKTM WITH MEDICAL SPECIMENS |
| VEGETATION | SIZE OF SOFT DRINK CAN | BEST SAMPLES DEPEND ON THE AMOUNT OF CONTAMINATION FOUND NEAR THE RELEASE POINT |
| MEDICAL SPECIMENS | ||
| URINE | 20 TO 50 ML | MUST OBTAIN CONSENT TO COLLECT SPECIMENS FROM OTHER THAN US CASUALTIES |
| WHOLE BLOOD OR SERUM | 5 ML | MUST OBTAIN CONSENT TO COLLECT SPECIMENS FROM OTHER THAN US CASUALTIES |
| CEREBRAL SPINAL FLUID | 2 ML | MUST OBTAIN CONSENT TO COLLECT SPECIMENS FROM OTHER THAN US CASUALTIES |
| ORGAN TISSUE | 30 G (MINIMUM) | |
| MEDIASTINAL LYMPH NODES | 2 | SHOULD BE REMOVED BY A SURGEON DURING AN AUTOPSY |
Although a sample/specimen collected from an alleged attack area can be significant, it can become useless if proper steps are not taken to record critical information about its collection or if it is improperly packed and breaks during shipment to an analysis center. This section discusses the information needed when acquiring samples/specimens and the preferred methods for handling and packing samples/specimens for shipment.
a. A complete background information history of the circumstances about each sample's/specimen's acquisition must be provided to the agency analyzing the sample/specimen.
b. Critical background information includes—
c. Provide information on the agent effects on vegetation for soil or environmental samples. A description of the general area (jungle, mountain, grassland) and changes in the vegetation after agent deposition (such as color change, wilting, drying, dead) in the main attack and fringe areas.
d. Provide information on the agent effects on humans for medical specimens. Describe how the agent affected personnel in the main attack area versus fringe areas; the duration of agent effects; peculiar odors that may have been noticed in the area prior to, during, and/or after an attack; measures taken that alleviated or deteriorated the effects; and the approximate number of victims and survivors, to include their ages and genders.
e. Describe the agent effects on animals. Provide information on the types of animals that were or were not affected by an attack and of how they were affected.
Materials used for packaging samples/specimens primarily consist of Mylar collection bags, TeflonTM specimen jars and tubes, pigs and piglettes, ice chests, sealing materials, and wrapping and cushioning supplies.
a. Collection Bag. Use the Mylar bag as the initial container for such samples as protective masks and filter canisters, individual antidote and decon kits, munition fragments, and other items too large to place in a specimen jar. Use it also to package sample/specimen containers to ensure a vapor barrier in case the container is broken in transit. The bag acts as an initial or secondary vapor barrier to prevent air from leaking inward and toxic material outward. Follow the procedures below when using the bag.
b. Glass Specimen Jars and Polypropylene Tubes. Use glass containers to hold small environmental samples, water samples, and medical and post mortem specimens. Use polypropylene containers to hold medical specimens such as blood or urine. Polypropylene containers may be used for post mortem specimens if required; however, glass containers are preferred. The use of glass rather than plastic containers is preferred for environmental samples because toxic agents may leach chemicals from plastics into a sample, introducing contamination and confusing the analysis efforts.
c. Six-Pound Metal Can. Use metal cans as the external container for packaging small items that have been sealed in Mylar bags, specimen jars, and polypropylene tubes containing medical specimens. The metal can helps absorb shock from rough handling during shipment and eliminates the spread of contamination if a specimen container is broken. The six-pound metal can is capable of holding more than one sample/specimen (depending upon size of samples/specimens).
d. Ice Chest. Standard polyethylene or metal ice chests are the most easily procured items used for transworld shipment of CB samples/specimens. The most easily used size is about 24 inches long by 18 inches high by 15 inches deep. This size permits the sender to ship two or three 6-pound metal cans in each chest with sufficient dry ice to maintain freezing temperatures for about four days. Also, each chest remains at a weight that one individual can handle.
e. Transport Container. When the samples/specimens must be transported on commercial aircraft, an IATA-approved sample transport container must be used for shipment/delivery to the CONUS laboratory.
f. Coolants. Samples/specimens submitted for laboratory analysis must be properly packaged, labeled, and shipped to ensure they arrive in an analytically acceptable condition. All samples should be maintained at a temperature of 1° to 4°C during transport. Ideally, samples/specimens should arrive at the in-theater laboratory within 6 hours of collection. The samples/specimens should be delivered to the CONUS laboratory within 24 to 48 hours. If the samples/specimens cannot be delivered to the CONUS laboratory within this time, then they should be flash frozen to -165°C, if capabilities are available. If available, dry ice should be used when flash freezing cannot be accomplished. If the samples/specimens cannot be delivered to the CONUS laboratory within 24 hours, the supporting laboratory should subculture the samples/specimens and send the subculture with the samples/specimens to the CONUS laboratory. The subculturing date should also be provided.
g. Internal Insulation. While a commercial ice chest provides good insulation of both the samples/specimens and the coolant, it is best to place extra insulation and cushioning around the metal cans inside the chest. Newspapers, plastic bubble wrap, and foam rubber may all be used with almost equally good results except newspapers and standard ice do not mix well.
a. The collector must provide a formatted message for transmission as soon as possible to report acquisition and shipment of samples/specimens. During special operations in a theater in which a Special Forces Group (SFG) is deployed, the message is transmitted by the fastest means through the fewest channels to the NBC control (NBCC) center. If a NBCC center has not been deployed to the area of operations, as in low-sample/specimen volume peacetime NBC sampling operation, the message is [B-24] transmitted by the fastest means through the fewest channels to the message addressees below. In addition, a written report accompanies each sample/specimen or batch of samples/specimens. The collector ensures that the acquisition message has been properly classified.
b. The collection report includes at least the following addressees:
SECSTATE WASHDC
SECDEF WASHDCHOSD-ISA/OUS-DREH
JCS WASHDC//J-3/J-5H
CIA WASHDCHOSWR-STD-LSBNIC-NIO(STP)H
DIA WASHDC//DT-3B/DT-5A//
DIR AFMIC FT DETRICK MD//AFMIC-CR/AFMIC-SA//
DA WASHDC//DAMI-FIT/DAMO-SWC//
CMDT USACMLS FT LEONARD WOOD MO//ATSN-CM-CO//
CDR SBCCOM APG
MDHSMCCR-OPF/SMCTE-OPE-RA-ID2H
CDR FSTC CHARLOTTESVILLE VA//AIAST-RA-ID2H
CDR USAMRIID FT DETRICK MD (For suspect biological samples/specimens only.)
c. A collection message contains the following information:
d. Ship all samples/specimens by the fastest, safest means, preferably by a technical escort unit (TEU) to the theater Chemical-Biological Sampling Control Element (CBSCE) or to a location the CBSCE designates. If there is no CBSCE in the theater, send the samples/specimens IAW preplanned instructions from the Chemical-Biological Sampling Control Center (CBSCC) at CBDA, Aberdeen, Maryland. The CBSCC uses the following criteria to determine the final destination of each sample:
(1) In any case, the NBCC center must be notified in advance of shipment of the sample so additional instructions or deviations from standard instructions can be given. Figure B-l shows an example of a shipping notification message. The NBCC center will direct, in advance, that samples be sent to one or more of the following locations, depending on the category of the samples. Prior to shipment of samples/specimens, contact must be made with—
Commander
Technical Escort Unit
ATTN: SMCTE-OPE
Aberdeen Proving Ground, MD 21010
DSN: 584-4381 (Duty hours) DSN: 584-2773 (After duty hours)
(2) This unit controls the transport of samples/specimens to their final destination(s). Do not ship suspected toxic samples/specimens or munition systems to CONUS technical centers or intelligence agencies without coordination and prior approval by the recipient.
NOTE
Suspect CB samples/specimens are first delivered to the supporting medical laboratory in the AO for in-theater analysis before they are transported out of the AO. The supporting laboratory will withdraw an aliquot of selected samples/specimens for analysis. The supporting medical laboratory is responsible for providing the AO commander confirmatory identification within the AO. The CONUS-based reference laboratory is responsible for providing confirmatory identification for President and Secretary of Defense purposes.
FM AMEMBASSY DDTTTTZ JAN 02
TO CDR TEU APG MD//SMCTE-OPE//
SECSTATE WASHDC
SECDEF WASHDC//OSD-ISA/OUS-DRE//
INFO CIA WASHDC//OSWR-STD-LSB/NIC-NIO(STP)//
JCS WASHDC//J-3/J-5//
DIA WASHDC//DT-3B/DT-5A//
DIR NSA FT MEADE MD
DIR AFMIC FT DETRICK MD//AFMIC-CR/AFMIC-SA//
DA WASHDC/DAMI-FIT/DAMO-SWC//
CDR FSTC CHARLOTTESVILLE VA//AIAST-RA-ID2//
CDR CBDA APG MD//SMCCR-OPF//
CDR USACMLS FT MCCLELLAN//ATZN-CM-CU//
CLASSIFICATION
SECSTATE FOR...
SECDEF FOR...
CIA FOR...
JCS FOR J-3/J-5 FOR...
DA FOR DAMO-SWC FOR...
AFMIC FOR...
CBDA FOR FIO...
FSTC FOR AMXST-FW...
USACMLS FOR THREAT MGR...
E.0.12356: DECL: OADR (Note: This is included if the message is classified.)
TAGS: ...
Subject: Shipment of CB Samples/Specimens
REF(S): TEU MSG #, (DTG DDTTTT [time zone] JAN 02)
1. (W) SHIPPING INFORMATION:
2. SPECIAL HANDLING REQUIREMENTS: DRY ICE ENCLOSED AS COOLANT.
3. SHIPMENT CONSISTS OF TWO ICE CHESTS (1 FOR CRDEC AND 1 FOR AFMIC) CONTAINING SIX SAMPLES/SPECIMENS.
ALL LIQUID SAMPLES/SPECIMENS ARE IN POLYPROPYLENE TUBES AND HAVE BEEN CAREFULLY PACKED TO AVOID
BREAKAGE. THE FOLLOWING SAMPLES ARE INCLUDED IN THE SHIPMENT:
| SAMPLE/SPECIMEN NUMBER | MESSAGE REFERENCE |
| TH-850102-001AG THRU TH-850102-005AG | BANGKOK DDTTTTZ JAN 02 |
4. USDAO HAS STATED THAT THIS SHIPMENT IS PARTIAL FULFILLMENT OF CIR.
Figure B-l. Sample shipping notification message.
The sample/specimen background document allows a collector to note the most relevant details associated with pre- and post-sample/specimen collection conditions. Do not consider the report to be all-inclusive. The information collected should include at least the items listed in Figure B-2. Interviews should be conducted with individuals exposed to the CB agent as well as individuals not exposed (see Figure B-3).
1. ID NUMBER___________
2. COLLECTION (DATE/TIME):______________________
3. COLLECTOR/UNIT:_______________________________
4. TYPE: ENVIRONMENTAL___ BIOMEDICAL___ SINGLE___ MULTIPLE___
5. PURPOSE: ATTACK___ CHEM/BIO ALARM___ CHEM DETECT___ RECON ILLNESS/DEATH___ OTHER___
6. POSTEXPOSURE: HOURS___ DAYS___ WEEKS___ UNKNOWN___
7. LOCATION: TOWN_________________ COORDINATES____________________________
A. TERRAIN: FLAT___ HILLS___ MOUNTAIN___ DESERT___ JUNGLE___ SPARSE TREES___ GRASS___ BODY OF WATER/TYPE___
B. WEATHER: CLEAR___ CLOUDY___ RAIN___ FOG___ SNOW___ DUST___
C. WIND: LIGHT___ HEAVY___ GUSTY___ NONE___
D. ODOR: SWEET___ FRUITY___ PEPPER___ FLOWER___ IRRITATING___ CHANGING___ NONE___ OTHER_____
E. TEMPERATURE AT TIME OF ATTACK:_____ TEMPERATURE AT TIME OF SAMPLE COLLECTION:_____
8. COMMENTS: _____________________________________________________________ _____________________________________________________________
9. ATTACK: DATE/TIME________ METHOD: ARTILLERY___ ROCKET___ AIRCRAFT___ MORTAR___ RPG/GRENADE___ OTHER, DESCRIBE:______________________________
A. EXPLOSION: AIR_________ (HEIGHT)_________ GROUND___________ SIZE_______ DISTANCE_________ DESCRIBE:____________________
B. CONSISTENCY: SMOKE___ MIST___ DUST___ RAIN___ GEL___ INVISIBLE, DESCRIBE:_______
10. ENVIRONMENTAL SAMPLE: SOIL___ WATER___ VEGETATION___ AIR___ OTHER___
11. BIOMED SPECIMEN: ACUTE___ CONVALESCENT___ EXPOSED___ NOT ILL___ POSTMORTEM___ CONTROL, EXPLAIN:___________________ BLOOD___ LIVER___ LUNG___ SPLEEN___ BRAIN___ SKIN___ KIDNEY___ URINE___ OTHER, DESCRIBE:_________________________
12. COMMENTS: _________________________________________________________
13. CASUALTY: SSN_______________ UNIT_______________________ SEX______
[B-28] 14. SIGNS/SYMPTOMS: ONSET__ DURATION__
A. HEAD: FEVER___ CHILLS___ HEADACHE___ FLUSHED___ DIZZINESS___ UNCONSCIOUSNESS___ COMA___ HALLUCINATIONS___
B. EYES: SUNLIGHT SENSITIVE___ PAINFUL___ BURNING___ DROOPY EYELIDS___ DOUBLE VISION___ BLURRED VISION___ LARGE PUPILS___ PINPOINT PUPILS___
C. NOSE: RUNNY___ BLEEDING___
D. THROAT: SORE___ DRY___ SALIVATING___ BLOODY SPUTUM___ HOARSENESS___ DIFFICULTY SPEAKING___
E. RESPIRATION: DIFFICULTY BREATHING___ CHEST/PAIN DISCOMFORT___ WHEEZING (IN/OUT)___ COUGHING___ LABORED BREATHING___
F. HEART POUNDING OR RUNNING___ IRREGULAR HEARTBEAT___
G. GI: LOSS OF APPETITE___ NAUSEA___ FREQUENT VOMITING___ FREQUENT DIARRHEA___ VOMITING BLOOD___ DIARRHEA WITH BLOOD___
H. URINARY: BLOODY URINE___ UNABLE TO URINATE___
I. MUSCULOSKELETAL: NECK PAIN____ MUSCLE TENDERNESS___ MUSCLE TREMBLING/ TWITCHING___ WEAKNESS___ PARALYSIS, DESCRIBE:_____________________ CONVULSIONS___ TREMORS___ MUSCLE ACHES___ BACK PAIN___ JOINT PAIN___
J. SKIN: RASH___ REDDENING___ ITCHING___ BLISTERS___ PAIN___ NUMBNESS___ PROFUSE PERSPIRATION___
15. COMMENTS:
_____________________________________________________________
16. ANIMALS AFFECTED: YES___ NO___ DESCRIBE:_________________
17. RELATED SPECIMENS_________________________________________
ID NUMBER_____________________________________
DESCRIPTION__________________________________
18. COLLECTOR
SIGNATURE____________________________
NAME___________________________
PHONE NUMBER______________________
E-MAIL________________________
19. REVIEWER
SIGNATURE
NAME
PHONE NUMBER_________________
E-MAIL_________________
Figure B-2. Sample/specimen background document.
CB INCIDENT INTERVIEW
DATE:_____________________ INTERVIEWER: _____________________________
SUBJECT'S NAME: _____________________________________________________
ALIAS #1_____________________________ #2_____________________________
AGE:____________ SEX: ____ M ____F YEAR OF BIRTH:_________
NATIONALITY:______________________________________________
SUBJECT'S ADDRESS:_________________________________________
IDENTITY CARD #:___________________________________________
DELIVERY METHODS:
TYPE:____UNKNOWN _____GROUND ____AIR _____ARTILLERY/ROCKET ____MINE
OTHER, DESCRIBE:_______________________________
HEIGHT: ______(M)
SIZE:_____________ (AFFECTED AREA IN METERS)
DISTANCE:__________(M)
AGENT CHARACTERISTICS
ODOR: _____NONE _____SWEET _____FRUITY _____IRRITATING _____PEPPER
_____FLOWER _____CHANGING _____
OTHER, DESCRIBE:___________________________
COMMENTS:____________________________________________________
_____________________________________________________________
CONSISTENCY:
______SMOKE ______MIST _____DUST _____RAIN ______GEL ______DRY
____VISIBLE ____INVISIBLE ____OTHER, DESCRIBE:_______________________
COLOR:_____________ DESCRIBE DEVELOPMENT OF COLOR:___________________
AREA COVERAGE:______________________________________________
PHYSICAL DISSEMINATION/COVERAGE (i.e., DROPLET SIZE AND DISTRIBUTION):
WRITE OR DRAW______________________________________________
SYMPTOMS:__________________________________________________
_____________________________________________________________
INDIVIDUAL'S ACTIONS:
DURING ATTACK:_____________________________________________
___________________________________________________________
AFTER ATTACK:______________________________________________
___________________________________________________________
PROTECTIVE MEASURES:_______________________________________
TREATMENT RECEIVED:________________________________________
___________________________________________________________
ENVIRONMENTAL EFFECTS: VEGETATION CHANGE? ___YES ___NO
DESCRIBE:__________________________________________________
___________________________________________________________
ANIMALS AFFECTED? ___YES ___NO
DESCRIBE:__________________________________________________
OTHERS AFFECTED:
NAME AGE SYMPTOMS RESOLUTION
___________________________________________________________
___________________________________________________________
___________________________________________________________
Figure B-3. Chemical/biological incident interview.
FM AMEMBASSY DDTTTTZ JAN 02
1.00
SHIPPING
TO CDR TEU APG MD/SMCTE-OPEI/
SECSTATE WASHDC
SECDEF WASHDC//OSD-ISA/OUS-DRE//
INFO CIA WASHDC//OSWR-STD-LSB/NIC-NIO(STP)//
JCS WASHDC//J-3/J-5//
DIA WASHDC//DT-3B/DT-5A//
DIR NSA FT MEADE MD
DIR AFMIC FT DETRICK MD//AFMIC- CR/AFMIC-SA//DA WASHDC//DAMI-FIT/DAMO-SWC//
CDR FSTC CHARLOTTESVILLE VA//AIAST-RA-ID2//
CDR CRDEC APG MD//SMCCR-OPE//
CDR USACMLS FT LEONARD WOOD MO//ATSN-CM-CO//
CDR USAMRIID FT DETRICK MD// (FOR SUSPECT BIOLOGICAL SAMPLES/SPECIMENS ONLY)
CLASSIFICATION
SECSTATE FOR...
SECDEF FOR
CIA FOR
JCS FOR J-3/J-5 FOR
DA FOR DAMO-SWC FOR
AFMIC FOR
CRDEC FOR FIO
FSTC FOR AMXST-FM
USACMLS FOR THREAT MGR
E.O. 12356: DECL: OADR (NOTE: This is included if the message is classified.)
TAGS:
SUBJECT: SHIPMENT OF CB SAMPLES
REF(S): TEU MSG, #______. (DTG DDTTTT [time zone] Jan 02)
1. INFORMATION:
2. SPECIAL HANDLING REQUIREMENTS: DRY ICE ENCLOSED AS COOLANT.
3. SHIPMENT CONSISTS OF TWO ICE CHESTS (1 FOR CRDEC AND 1 FOR AFMICO) CONTAINING SIX SAMPLES/SPECIMENS. ALL LIQUID SAMPLES/SPECIMENS ARE IN POLYPROPYLENE TUBES AND HAVE BEEN CAREFULLY PACKED TO AVOID BREAKAGE. THE FOLLOWING SAMPLES/SPECIMENS ARE INCLUDED IN THE SHIPMENT:
| SAMPLE/SPECIMEN NUMBER | MESSAGE REFERENCE TH-8501 |
| 1AG THRU TH-850102-005AG | BANGKOK DDTTTTZ JAN 0202-00 |
4. USDAO HAS STATED THAT THIS SHIPMENT IS PARTIAL FULFILLMENT OF CIR.
As the HSS unit prepares for its support role, NBC, TIM, and CBRNE considerations must be included. This appendix provides guidelines for HSS planning, preparing for, and conducting operations in an NBC environment and responding to a homeland defense CBRNE event.
When preparing the unit's mobilization plan and TSOP, include the supplies and equipment that will be required for the unit to operate in an NBC environment. DO NOT wait until ordered to mobilize to begin preparation for the mission. A well-prepared and trained unit stands a much better chance of surviving and accomplishing their assigned mission. At a minimum include the following:
During mobilization the unit must ensure that all supplies and equipment are on hand and are serviceable. Commanders and leaders must also ensure that—
Plans for establishing a BAS, DCS, or FST for operating in a NBC environment must include employment of CBPS systems. When establishing a hospital using Deployable Medical Systems (DEPMEDS), the chemically protected (CP) DEPMEDS must be set up as the conventional shelters are being set up. Once the conventional shelter has been set up and is operational, CP DEPMEDS cannot be established without first taking down the existing shelter. Follow the technical manual provided with the CP DEPMEDS system issued to your unit. Plans for operating a DEPMEDS equipped hospital in the NBC environment should include, but not be limited to—
Individuals should have decontaminated themselves or have been decontaminated by unit personnel; however, an MTF must plan for and be prepared to receive contaminated patients. The patients may not have been decontaminated at the unit, or they may have become contaminated en route to the MTF. Selected CSHs may be designated as the primary NBC MTF and be augmented with additional supplies and medical staff. When designated as such, plans must be prepared designating the location of the CSH that can best support the forward deployed MTFs. All actions listed in paragraph C-4 must be taken. During operations, actions that must be taken are—
Plans for providing preventive medicine services must include monitoring water supplies for contamination. To perform this mission, equipment and supplies must be available and operational. Essential equipment and supplies include—
Plans for veterinary services must include provisioning for treatment to government-owned animals and quality control of food supplies. To perform their mission, essential supplies and equipment include—
Most dental services at the dental treatment facilities will have to be suspended in NBC contaminated areas due to a lack of CPS. Plans must include for emergency dental services to be provided in a clean area or in an MTF with a CPS. Essential supplies and equipment include—
Although specific supplies and equipment are not required for COSC, plans must be prepared to provide these services under NBC conditions. The COSC staff must locate clean areas to conduct COSC activities or manage the COSC patients in a MOPP level commensurate with the command MOPP guidance.
Planning for medical laboratory support must include plans for conducting analysis on suspect NBC samples/specimens. Designated supporting medical laboratories must be prepared to analyze and provide confirmation/identification on specimens/samples of suspect NBC agents from humans, water sources, food supplies, and the environment (air and soil). The samples/specimens may be collected by MTF personnel, chemical corps personnel, PVNTMED personnel, veterinary personnel, or other services personnel. To perform this mission, supplies and equipment should include—
Plans must include health service logistics support to continue under NBC conditions. To continue this role, all supplies must be protected from contamination. Materials required include—
NOTE
This guideline contains items that are required specifically for HSS operations in an NBC environment. The items are in addition to supplies and equipment required for conventional operations. This guideline is not all inclusive, but is a starting point for HSS units to develop their specific guidelines.
Health service support units and installation medical activities/centers must be prepared to provide support in the event that CBRNE are used on the United States. Medical commanders and leaders should develop plans on how the provision of medical support will be provided to a CBRNE event. The plan should include, but not be limited to—
The primary purpose of the Medical Planning Guide for the Estimation of Nuclear, Biological, and Chemical Battle Casualties—AMedP-8(A), a three-volume publication for NBC, is to assist medical planners, medical logisticians, and medical staff officers in predicting NBC warfare contingency requirements for HSS personnel, medical materiel stockpiles, patient transport or evacuation capabilities, and facilities needed for patient decontamination, triage, treatment, and supportive care. The optional use of these guides is for projecting medical NBC operational estimates at brigade, division, corps, and EAC.
NOTE
The use of "the guide" in this appendix refers to AMedP-8(A), Volume I, II, or III. The AMedP-8(A), Volume I, II, or III, is the text for each of the STANAGs. The contents of this appendix are extracts from Sections 1, 2, and 3 of the guide.
Medical planners' estimates (such as casualty, logistics, evacuation, and personnel cross leveling) must be modified for the NBC environment. Estimates of NBC medical workload can be found in AMedP-8(A). A compact disk containing these documents and an automated version of AMedP-8(A), the Casualty Requirements Estimation Tool (CREST), can be obtained from Headquarters, Department of the Army, ATTN: DASG-HCZ-FD, 5109 Leesburg Pike, Falls Church, VA 22041-3258. The CREST is primarily an Army tool focusing on corps, brigades, and battalions, but also models aerial ports of debarkation, seaports of debarkation, and other units.
This Section Implements STANAG 2475.
a. Volume I of the guide provides estimates of casualties and remaining operational strength after a nuclear detonation in a brigade-sized unit during an out-of-area contingency operation. These estimates [D-2] include the numbers, injury type (initial nuclear radiation, blast, and thermal injuries), and injury severity of nuclear patients based on several brigade scenarios. The scenarios include three different brigade-sized units, in warned or unwarned posture, which have single detonation of 5, 20, or 50 KT in the unit area.
b. The guide is organized into 10 sections. Section 1 introduces the guide and presents background and medical planning considerations. Section 2 provides information on the methodology used to develop the estimates of fatalities, casualties, and effectiveness of individuals remaining in the unit. Section 3 explores the use of the casualty prediction tables based on combat effectiveness decrements and estimates of the number of casualties categorized by insult level. Sections 4 through 10 contain tables of casualty estimates.
c. A sample of this information is graphically depicted in Tables 1-1 and 1-2 of the guide. The casualty estimates used to prepare these tables are presented in the guide as Tables 6-4 and 10-4 in Sections 6 and 10 respectively. The use of these tables is explained in paragraphs 3.1 through 3.6 of the guide. Paragraphs 3.7 and 3.8 of the guide discuss how to use the guide for situations not explicitly addressed.
d. The effects of residual radiation on personnel are not included in the guide. AMedP-6 and AMedP-7 provide information on planning, operations, and treatment for a residual radiation situation. Also not included is the impact of tumbling; impact of glass shards from windows of vehicles or buildings; crushing deaths from building failure; or COSC casualties; thus causing underestimations on the number of patients. Further, there will be personnel who get radiation doses or burns and do not seek medical care.
e. A nuclear detonation may introduce new levels of destruction to the battlefield. There is very little experience with nuclear effects and there is certainly no experience with these weapons on a modern, highly technological battlefield. Therefore, there is little historical data on which to base estimates of personnel injured. Computer simulations are generally used to estimate numbers of personnel injured. Although these estimates may include significant uncertainty, they provide the best estimates to date.
a. For effective mass casualty management, key medical and related considerations must be well planned and practiced. These include on-site triage and emergency care, communications, health service logistics, evacuation by ground and air resources, and personnel training in self-aid/buddy aid. Plans need to be made for requirements that may differ from the usual combat situation. For example, in combat situations, severe burn injuries in large numbers are relatively uncommon. Therefore, no special planning for the care of large numbers of burn patients is required. In a nuclear environment, this may not be true, and consideration must be given to the increased need for medical support that would result from a high incidence of burn patients.
b. Prior to an attack, the data may be used by medical planners to augment the requirements for conventional combat as appropriate for the nuclear situation. The tables can be used to prepare estimates of the number of patients at all echelons.
c. After an attack, the effectiveness and adequacy of the medical support effort during the first 24 hours are critical. Commanders should be informed rapidly of the estimated medical load in order to provide rescue and treatment resources or request assistance from higher headquarters, adjacent units, or allied units. These estimates should be updated postattack based on aerial or ground reconnaissance and survey.
d. In addition to casualties, a nuclear weapon detonation can generate an EMP that may cause catastrophic failures of electronic equipment components and may adversely affect the capability of all units in the area of the detonation. Electromagnetic pulse has no direct effect on personnel and is not further addressed in this publication.
Since a nuclear detonation may produce mass casualties, plans for a triage system must be in place. Paragraphs 3.4 through 3.5 of the guide describe patient categories by injury severity and may be used to estimate the number and injury severity of patients for a particular operational scenario. The guide does not, however, provide estimates of the number of patients by triage classification.
a. An efficient and flexible evacuation plan is absolutely essential for the preservation of life and to retain the mobility of forward medical resources. In a potential mass casualty situation, the full range of evacuation assets should be considered.
b. The extended hospital time of nuclear casualties will influence levels of evacuation or hospitalization. In addition, estimates of the different types of casualties can be a consideration in evacuation planning. In planning for evacuation, estimates provided in the guide can be used as a starting point from which to estimate evacuation resources.
a. Some personnel within the military unit may not be classified medically as casualties, but will require some self-aid and buddy aid. A casualty is defined as anyone entering the medical system. Paragraph 2.5 of the guide further describes the basis for casualty calculation.
b. Nuclear detonations will produce a large number of blast, burn, and projectile injuries that initially must be treated by individual soldiers trained in first aid procedures. The physical damage to the surrounding area as a result of a nuclear detonation will increase delays in medical assistance and evacuation. Training in self-aid/buddy aid will improve casualty survival rates and conserve medical resources. The guide can be used to provide a conservative estimate of the numbers of injured that will require first aid. The tables in Sections 4 through 10 of the guide, showing the status of unit personnel by time period, can be used to indicate the numbers of personnel who are injured (but not casualties) who may require first aid.
The data provided in the guide can be used to determine immediate additional bed requirements resulting from a nuclear detonation. In addition to the numbers of patients who will need beds, the data provided in the guide can also indicate the increased hospitalization time of nuclear casualties. Long-term bed requirements, greater than 30 days, are not provided. Based on the theater evacuation policy specified for the operation, the hospital bed days may be in theater or in CONUS.
The data provided in the guide can assist in estimating the needed supplies. The supply system must be prepared for increased demands for certain types of medical and general supplies and equipment, kits, dressings, and antibiotics. The treatment of combined injuries will not require any special types of supplies, although demands for certain types of supplies will increase.
The assignment of medical support is normally based upon the total military population and the expected conventional casualty rate. The data provided in the guide may be used to assess the requirement for additional medical units. The planning guidance presented in this document can (and should) be modified to reflect the needs of the anticipated operation, including operational tempo, national/coalition priorities, medical resource allotment, and so forth. When trying to augment personnel, consider that the use of a nuclear weapon in a tactical situation could be an indication of an increased tempo of warfare. Therefore, even though a unit may be targeted with a nuclear detonation, that unit may not be the site where the highest numbers of casualties are being produced, and another unit may have priority of support.
This Section Implements STANAG 2476.
The guide, AMedP-8(A), Volume II, provides estimates of casualties, and remaining operational strength, after single BW attacks on tactically deployed, brigade-sized land force units, offshore naval and marine forces, and selected strategic targets in rear areas. These worst-case casualty estimates are for personnel [D-5] within both the targeted and the downwind hazard areas of the attacked forces. They assume that all affected personnel will be unsheltered and unwarned. To further estimate worst-case outcomes, the guide assumes that exposed individuals have not been vaccinated against any of the evaluated agents, nor have they undergone any type of medical prophylactic treatment prior to exposure. The tables included in the guide are designed to show numbers of expected casualties; expected fatalities; personnel at different performance levels; and times after exposure. In selected scenarios, the guide provides a method for estimating casualties among collocated civilians based on local population density.
a. The guide presents casualty estimates for all possible combinations of the following conditions:
b. The guide is subject to limitations of extent and content. Since there are many more possible attack variables than those considered, the guide presents a limited number of estimates and provisional guidance for estimating cases not modeled. These estimates are based upon the best available medical data, but such data result in qualified estimates. Therefore, for more authoritative medical descriptions, medical planners and staff personnel should use FM 8-9, NATO Handbook on the Medical Aspects of NBC Defensive Operations, AMedP-6(B), Part II—Biological. Users of the guide must amplify or modify these estimates to meet emergent requirements such as injuries resulting from combined biological and conventional attacks.
c. Computer models that integrate available information have been used to predict the effects of future biological attacks. These resultant estimates may include substantial uncertainties when applied to specific situations. However, they provide the best estimates available to date.
d. The guide is also organized into 10 sections. Section 1 introduces features of the guide, and then presents background and medical planning considerations. Section 2 provides information on the methodology used to develop the estimates. Section 3 describes how to use the tables presented in the guide. Sections 4 through 10 of the guide contain tables of casualty estimates, with one section for each of the seven biological agents.
e. Biological attacks are likely to have a significant impact on the medical system. As detailed elsewhere in the guide, victims may number in the hundreds or even thousands. Demand for medical care may quickly overwhelm available resources; this problem will be exacerbated if medical personnel themselves become victims of the attack. Local civilian populations will be victimized as well, limiting host-nation support and potentially adding to the demands on the military medical system.
f. A variety of medical responses to BW attacks are available, depending on the agent used and whether medical countermeasures are employed prior to attack or after exposure has already occurred. [D-6] For many agents, immunization or pre-exposure prophylaxis with antibiotics may prevent illness in those subsequently exposed. After exposure, disease can often be prevented or ameliorated via immunization and therapeutic use of antibiotics, antiviral drugs, and hyperimmune gammaglobulins.
a. Effective mass casualty management requires careful planning. The guide is designed to support such planning by providing medical planners and staff personnel with a systematic means for estimating the number of biological casualties. However, casualty management also involves practice of self-aid and buddy aid, on-site triage and emergency care, decontamination, transport to medical facilities, infection control measures, communications, health services, logistics, and evacuation by ground or air transportation.
b. Medical requirements resulting from attacks with biological agents may be substantially different from those resulting from conventional, nuclear, or chemical combat. There would be no indication of the presence of biological agents in most tactical situations. Units downwind from an attack area may be unexpectedly exposed to biological agents. In some cases, there will also be a risk of secondary infection and subsequent epidemics amongst troops and/or the local population. Additionally, use of biological agents may generate reservoirs within the local animal population that may serve as a further source of infection.
c. Often the first indication of an attack with a biological agent will be the development of symptoms in exposed personnel. Diagnosis and treatment are complicated by the fact that many of the agent-induced diseases described in the guide begin with symptoms associated with common illnesses, such as influenza. In such cases, biological agent attacks may generally be distinguished from naturally occurring epidemics by the sudden onset of disease, the large number of personnel presenting with similar symptoms, and the concentration of those personnel in geographically contaminated areas.
a. Since a biological attack may produce mass casualties, preparations for a triage system should be in place before the attack. Paragraph 3.3.8 of the guide describes patient categories by illness severity. For a particular described operational scenario, this information may be used to estimate the number of patients with specified levels of illness. The guide does not provide estimates of the number of patients by triage classification or usual medical descriptions.
b. Decontamination of patients must be considered before further evacuation.
a. An efficient and flexible evacuation plan is essential for adequate casualty treatment and to retain mobility of forward medical resources. For an assessment of a potential mass casualty situation, the medical planner should consider the full range of evacuation assets, limitations, and obstacles. After an [D-7] attack, the medical staff may need to estimate the number of casualties that could require evacuation at given postexposure times.
b. Evacuation requirements will vary with the type of biological agent used. Casualties resulting from some agents may not be evacuated because the time course of effects is relatively short. For others, like botulinum toxin, casualties may require evacuation to a facility where they can receive care for weeks or even months. Estimates provided in the guide can be used as a starting point from which to plan for evacuation resources, including those required for decontamination of personnel and transportation assets.
The casualty estimates in the guide are presented without allowance for in-unit care. However, there may be need for rapid intervention. Delays in obtaining medical care may occur because of physical damage or contamination of the surrounding area. Soldiers trained in first aid procedures may be the first to provide aid to biological agent casualties. The guide provides a conservative estimate of the numbers of exposed personnel who will require first aid. The tables described in paragraphs 3.3.2 through 3.3.4 of the guide give the time courses of effects that may apply to estimation of in-unit care and delayed medical requirements.
Bed requirements can be estimated using the tables described in paragraphs 3.3.2 through 3.3.4 of the guide. The latter type of table is useful after an attack since it shows gains and losses of casualties over time. The type of table described in paragraph 3.3.5 of the guide may be more useful for long-range planning. It shows maximum numbers of personnel by illness severity category. The tables in the guide only provide estimates for the first 35 days after attack. Based on the theater evacuation policy specified for the operation, hospital days may be in theater or in the national area.
a. The estimates provided in the guide are intended to support projections of medical materiel and logistical requirements. Increased demands may occur for certain types of medical and general supplies, including equipment, kits, antibiotics, disinfectants, and other critical medical materiel. Demands may also increase for items unique to the prevention and treatment of biological agent casualties, such as vaccines, antibiotics, and antisera, as well as items adapted to contaminated environments. Tables showing maximum numbers of personnel by illness severity category can provide useful input for logistical planning.
b. Often the first indication of an attack with a biological agent will be the development of symptoms in exposed personnel. Diagnosis and treatment are complicated by the fact that many of the agent-induced diseases described in the guide begin with symptoms associated with common illnesses, such as influenza. In such cases, biological agent attacks may generally be distinguished from naturally occurring diseases.
a. The assignment of medical support is normally based upon the total military population and the expected conventional casually rate. The guide may be used to assess requirements for additional medical units.
b. Although a specific unit may be the target of a biological attack, more casualties could be suffered by other units downwind. Accordingly, a unit other than the targeted one may have priority for support. The tables presented in the guide can be used in planning for either situation. Some tables show estimated maximum numbers of personnel by illness severity category. Such estimates should be combined with a comprehensive array of other available information to increase the effectiveness of medical force planning.
This Section Implements STANAG 2477.
a. The primary purpose of Volume III is to assist medical planners, logisticians, and staff officers in predicting CW contingency requirements. Requirements include medical personnel, medical materiel stockpiles, patient transport or evacuation capabilities, and facilities needed for patient decontamination, triage, treatment, and supportive care. An optional purpose is to support medical operational estimates.
b. The guide provides medical worst-case estimates of casualties and remaining operational strength after a single CW attack on a tactically deployed, brigade-sized land force units, with protection available and protection unavailable. These worst-case casually estimates are for personnel located within both the targeted and the downwind hazard areas of the brigade. It is assumed that all targeted personnel will be unsheltered and without medical pre-exposure prophylactic treatment. Tables in the guide are designed to show total numbers of—
c. The guide presents estimates of personnel status at specific time points. These range from 1 to 3 hours to 7 to 30 days after an attack, depending on the type of agent considered. Such estimates are projected from all possible combinations of the following conditions:
d. An index to essential information and four sample problems to illustrate use of this information are at the end of the guide (see Section 11). Section 11 provides a planning guide overview, describes applications, and presents a brief explanation of modeling methods used to prepare estimates.
e. The guide is subject to limitations of extent and content. Since there are many more possible attack variables than those considered, the guide presents a limited number of estimates. These estimates are based upon the best available toxicological values, but such values are qualified estimates. Therefore, medical planners and staff personnel should use FM 8-9, NATO Handbook on the Medical Aspects of NBC Defensive Operations, AMedP-6 (B), Part III—Chemical, for more authoritative medical descriptions and information on effects of longer duration.
f. The guide is most value to the user who needs to know what kinds of casualties to expect, relative numbers of each, and the time frames in which they are likely to appear. To assist the user, who lacks experience in actual CW, the guide describes types of injury, relevant factors, general magnitudes of effects, and effects of time courses on chemical casualty numbers. The casualty estimates are appropriate for training exercises. However, this initial attempt to provide complex estimates has limitations for battlefield use. The limitations are described as follows:
a. The guide provides medical planners and staff personnel with a systematic means for estimating chemical casualties in various-sized units, without regard to composition. This document provides more accurate and detailed estimates and is based upon detailed operational scenarios for brigade-sized units. Both chemical planning guides support estimates of combat performance from individuals remaining in the unit.
b. Effective mass casualty management requires careful planning. The guide is designed to support such planning by providing medical planners and staff personnel with a systematic means for estimating the number, type, and time-related status of chemical casualties.
NOTE
Each user is advised to consult any available national military NBC defense doctrinal publications of similar nature.
c. Medical requirements during CW may be substantially different from those for the usual combat situation. There may be no indication of the presence of chemical agents in some tactical situations. Unprotected units downwind from an attack area, or those entering contaminated areas in an unprotected posture, may be unexpectedly exposed to chemical agents. However, casualty management also involves practice of self-aid and buddy aid, on-site medical triage and emergency care, transport to medical facilities, communications, health services, logistics, and evacuation by ground or air transportation.
d. The signs and symptoms of chemical agent exposure may be sudden and intense, or delayed and subtle, depending on the agent used and the level of exposure. Individuals may not reach the first level of care for 15 to 60 minutes after the onset of effects. Decontamination may delay medical treatment. Stabilization should occur before casualties leave emergency care areas, but contamination of these areas may delay the stabilization process. However, effects of decontamination or secondary contamination on estimated doses and effects are not considered in the guide. For medical planning, users of the guide need to consider the various qualifications of its casualty estimates, as discussed in paragraphs 3.4 and 3.4.2 of the guide.
e. A chemical burn caused by HD can require more care than a same-sized burn induced by conventional munitions. Therefore, the initial prognosis may require revision after treatment is underway, and estimates of percent capable by performance band may require adjustment.
Since a chemical attack may produce mass casualties, preparations for a triage system should be in place before the attack. Paragraph 2.5.1 of the guide describes patient categories by injury severity. For a particular described operational scenario, this information may be used to estimate the number of patients with specified levels of injury. The guide does not provide estimates of the number of patients by triage classification or usual medical and toxicological descriptions.
a. An efficient and flexible evacuation plan is essential for adequate casualty treatment and to retain mobility of forward medical resources. For assessment of a potential mass casualty situation, the full range of evacuation assets, limitations, and obstacles should be considered by the medical planner. After an attack, the medical staff may need to estimate the number of casualties that require evacuation resources at given postexposure times.
b. Evacuation requirements will vary with the type of chemical agent used. Nerve agent casualties may not be evacuated because the time course of severe effects is relatively short. Depending upon exposure conditions, HD casualties may or may not require evacuation to a facility where they can receive care for several days, or possibly 6 to 9 months. Estimates provided in the guide can be used as a starting point from which to plan for evacuation resources.
The casualty estimates in the guide are presented with no allowance for in-unit care such as self-aid or buddy aid. Soldiers trained in first aid procedures may be the first to see chemical injuries. The guide can provide an estimate of the numbers of injured personnel who will require first aid. However, there may be need for rapid augmentation, support, or other intervention. Delays in obtaining medical care may occur because of physical damage or contamination of the surrounding area. The tables described in paragraphs [D-12] 3.3.2 and 3.3.3 of the guide give the time courses of effects that may apply to estimation of in-unit and delayed medical requirements.
Requirements for patient beds and hospitalization time may be greater after chemical exposures than after a conventional attack. Such increases are particularly important for agents, such as HD, that produce injuries followed by a long recovery period. Bed requirements can be estimated using the tables described in paragraphs 3.3.2 and 3.3.3 of the guide. Casualties Occurring by Time Period tables (see paragraph 3.3.3) in the guide are useful after an attack since they show gains and losses of casualties over time. Personnel by Injury Category tables (as described in paragraph 3.3.4) in the guide may be more useful in long-range planning. They show maximum numbers of personnel by injury severity category. The tables in the guide only provide estimates for the first 30 days after attack. Depending upon the theater evacuation policy specified for the operation, hospital days may be either in theater or in the national area.
The estimates provided in the guide are intended to support projections of medical materiel and logistical requirements. Increased demands may occur for certain types of medical and general supplies. These may include specific equipment, kits, dressings, antibiotics, and other critical medical materiel. Demands may also increase for items unique to the chemical battlefield (such as nerve agent antidote autoinjectors), as well as items adapted to chemical environments (including IV systems and special self-contained intensive care units). Tables showing maximum numbers of personnel by injury severity category (see paragraph 3.3.4 in the guide) can provide useful input for logistical planning.
a. The assignment of medical support is normally based upon the total military population and the expected conventional casualty rate. The guide may be used to assess requirements for additional medical units. The use of chemical weapons in tactical situations could be one indication of an increased tempo of warfare and need for additional personnel.
b. Although a unit may be targeted for chemical attack, that unit might not be located where the highest number of casualties could occur (as in a downwind hazard area). Accordingly, another unit might have priority for support. The tables presented in the guide can be used in planning for either situation. Some tables (see paragraph 3.3.4 in the guide) show estimated maximum numbers of personnel by injury severity category. Such estimates should be combined with a comprehensive array of other available information to increase the effectiveness of medical force planning.
c. The guide is organized into 11 sections. Section 1 introduces the guide and presents background and medical planning considerations. Section 2 provides information on the methodology used to develop the estimates of fatalities, casualties, and effectiveness of individuals remaining in the unit. [D-13] Section 3 explores the use of the casualty prediction tables based on combat effectiveness decrements and estimates of the number of casualties categorized by insult level. Sections 4 through 10 contain tables of casualty estimates. Section 11 is a tutorial on use of the tool.
d. These medical worst-case casualty estimates (see paragraph 2.1.2 through 2.1.7 in the guide) are for personnel in the chemical-targeted and downwind hazard areas of the brigade sector. The actual areas presenting chemical agent hazards to personnel are relatively small and localized when compared to the entire brigade sector. These estimates are not valid for acute effects from repeated exposures, possible delayed effects of low dosage exposures, operational worst-case targeting, targets with different numbers or distributions of exposed personnel, or attacks involving different conditions (of meteorology, terrain, protective status, and so forth) than are modeled. Although the guide is primarily designed to support medical force planning for future CW defense, it may be used to anticipate short-term requirements. For example, delayed requirements of HD victims for care or evacuation resources may be predicted from tables that give estimates of casually numbers by injury type at given times after a CW attack (see paragraphs 3.3.2 and 3.3.3 in the guide).
1. PURPOSE. Establish standardized procedures for medical NBC staff officers planning, preparing for, detecting, reporting, and providing preventive/protective measures for NBC/TIM hazards. Establish planning procedures for conducting HSS in NBC/TIM environments. Also, establish procedures for providing technical guidance/support to leadership before, during, and after an NBC/TIM event.
2. PROCEDURES
a. Medical NBC staff officers prepare list of equipment and procedural guidelines for HSS operations under NBC/TIM conditions. (Provide a list of radiological detection devices, chemical agent detection/identification kits/devices, components of biological sample/specimen collection, and shipping containers. Provide guidelines/references for operating detection/identification devices.)
b. Planning actions for use before an NBC/TIM event. (Provide preventive/protective measures that the leadership can employ to reduce the health effects of a NBC/TIM event. Also, provide preventive/protective measures that leadership can employ to reduce the health effects of existing NBC/TIM hazards/contamination in an AO. Provide HSS leadership with procedures that can be employed to protect their unit and patients.)
c. Planning action for use during an NBC/TIM event. (Provide preventive/protective measures that the leadership can employ to reduce the health effects of a NBC/TIM event. Provide HSS leadership with procedures that can be employed to protect their unit and patients.)
d. Planning actions for use after an NBC/TIM event. (Provide preventive/protective measures that line leadership can employ to reduce/mitigate the health effects of an NBC/TIM event on the force. Provide HSS leadership with procedure that can be employed to mitigate the effects on their unit and patients.)
e. Planning actions for preventive medicine support for NBC/TIM events. (Provide types and numbers of PVNTMED units/personnel required to perform PVNTMED missions during such events. Describe mission requirements for units/personnel preparing for and reacting to the event. Describe types of samples required and how samples must be collected, preserved, packaged, and shipped to supporting medical laboratory for analysis. Describe detection/monitoring equipment required for the event; such as AN/PDR77, AN/VDR2 radiac meter, chemical agent monitor (CAM), and M272 water test kit.)
f. Planning actions for veterinary support for NBC/TIM events. (Provide types and numbers of veterinary units/personnel required to perform the veterinary service missions during such events. Describe mission requirements for units/personnel preparing for and reacting to the event. Describe types of samples/specimens required and how samples/specimens must be collected, preserved, packaged, and shipped to supporting medical laboratory for analysis. Describe food contamination and decontamination procedures. Describe detection/monitoring equipment required for the event; such as AN/PDR77, AN/VDR2 radiac meter, and CAM.)
g. Planning actions for medical laboratory support for NBC/TIM events. (Provide requirements for medical laboratory support for an NBC/TIM event. Describe types of laboratory test/procedures required to provide command verification on the use of an NBC device/weapon. Provide medical laboratory reporting requirements; example: provide report to command surgeon; Joint Task Force/theater commander; senior commander in affected operational area.)
h. Planning actions for combat health logistics support for NBC/TIM events. (Provide requirements for combat health logistics support units and personnel. Describe types of Class VIII supplies required to support HSS response to an event. Examples: Numbers of chemical agent patient decontamination MESs, chemical agent patient treatment sets, number of packets of chemical agent pretreatment tablets required, and chemoprophylaxis required for personnel exposed to a biological agent.)
i. Planning actions for combat stress control/mental health support for NBC/TIM events. (Provide requirements for COSC/mental health support units/personnel. Describe where and how COSC/mental health personnel will provide their support in response to the event.)
j. Planning for medical treatment of NBC/TIM event casualties. (Provide requirements for medical evacuation and treatment (including emergency dental care) support units/personnel. Provide requirements for nonmedical personnel to perform patient decontamination at the MTF. Describe where and how evacuation and treatment personnel will provide their support in response to include supervision of patient decontamination procedures.)
3. COORDINATION REQUIREMENTS. (Provide requirements for support such as who should transport/escort samples/specimens from unit of origin to support medical laboratory and on to the CONUS gold standard laboratory. Example: The Technical Escort Unit normally provides transportation and escort for suspect NBC samples, in their absence describe who will provide this service. Provide requirements for numbers of personnel required to perform patient decontamination at supporting MTFs. Describe decontamination support requirements for medical units; especially hospitals and major combat health logistics facilities.)
4. REPORTS. (Describe types of reports required and frequency of reporting on HSS aspects of NBC/TIM events. Reports should provide, at a minimum, aspects of event and recommended preventive/protective actions needed to prevent or minimize casualties.)
To continue the HSS mission under CB conditions, MTFs must search out contamination free areas or employ CPS systems. Levels I and II MTFs may be able to locate contamination free areas; however, due to the mobility limitations of hospitals, they must always be prepared to operate under CB conditions if the area is under attack. Systems that can be employed as an MTF (Levels I, II, III, and IV) are described in this appendix.
a. The CBPS system is employed at the BAS, DCS, and FST. The CBPS is attached to the hard-walled box on the rear of a high mobility multi-purpose wheeled vehicle (HMMWV). The BAS will have one CBPS system per treatment team; the DCS will have four CBPS systems; the FST will have three CBPS systems. Also, systems will be issued to other selected medical treatment teams. When employed at the DCS, the patient holding team will also require GP tents to hold their required number of patients (see Chapter 4). Patients held inside the CBPS will be those that have been decontaminated and admitted into the system for treatment and are recovering from the treatment procedures and are awaiting evacuation. Any patients held in the GP tent must remain in MOPP Level 4 (the GP tent will not have collective protection); these patients are those that are expected to RTD within 72 hours.
NOTES
1. Normally, patients will not be held at the DCS under NBC conditions unless evacuation cannot be accomplished. They should be RTD or evacuated to a clean MTF, as soon as the mission permits.
2. The CBPS can also be employed as the DCS in the conventional mode. Employment in either mode still requires GP tentage for patient holding to meet total patient holding requirements.
b. The DEPMEDS-equipped patient care areas of the US Army Force XXI hospital and the hospital unit base (HUB) of the Medical Force 2000 (MF2K) will employ the CP DEPMEDS. It will not protect personnel or patients from the thermal, blast, and initial radiation effects of nuclear weapons; however, it will provide some protection against fallout effects. Areas of the hospital that are not included in the chemically protected (CP) DEPMEDS are MF2K general hospital unit medical (HUM), MF2K field hospital unit holding (HUH), MF2K combat support and general hospital unit surgical (HUS), minimum care wards, administrative areas, food service, supply (including Class VIII), and staff quarters. The system includes—
c. The M20 simplified collective protection system is another system that is available. It consists of a chemically protected room liner, a CB filter blower, and an ambulatory air lock. However, it does not have a litter air lock making it unsuitable for litter patient care. The M20 may be used to protect medical staffs at the DCS, FST, and hospitals, patients held in the GP tents at the DCS and in the minimum care wards and staff quarters of the hospitals. Thus providing additional CB protection for staffs and patients.
To establish a BAS in a CBPS, use one CBPS per treatment team for conventional operations in a split-team mode. When operating in a squad configuration and in the conventional mode, the two CBPS systems may be complexed to provide more workspace. However, keep in mind that the treatment squad is not staffed to operate the two systems in the CB mode. Therefore, when the two systems are complexed and the treatment squad must convert and operate in the CB mode, they may want to close the complexing door and only use one system. When initially setting up the CBPS for operations in the CB mode, only one CBPS is setup; see Note 2 below. Set up the system as described in TM 10-5410-228-10. To be operational as a BAS, set up medical supplies and equipment as required or as designated in the TSOP. A PDS consisting of a contaminated ambulance point, contaminated triage point, a patient decontamination area, and a contaminated treatment area is established on the downwind (prevailing wind) side of the CBPS. An overhead cover of plastic sheeting (approximately 20 feet wide by 50 feet long) is set up over the PDS, the hot line, and the clean treatment/waiting area; the cover overlaps the air locks. The clean treatment/waiting area should have an area at least 20 feet wide by 15 feet long to allow space for placing patients into the litter air lock without crossing the hot line. A second area covered with 20 × 25 feet of plastic sheeting (the evacuation holding area) is set up beside the shelter on the opposite side from the generator. The clean treatment area is separated from the decontamination area by a hot line with a shuffle pit. Only clean [F-3] (decontaminated) patients or personnel are allowed to cross the hot line into the clean treatment area, or are admitted into the CBPS. Figure F-1 presents one layout of a BAS using the CBPS. See TM 10-5410-228-10 for complete details on setting up, operating, and maintaining the CBPS. Each CBPS provides 300 square feet of work area.
1. The overhead cover is not needed when the wind speed exceeds 10 knots per hour. The plastic will not stay in place.
2. Although each treatment team of the BAS has a CBPS; only one system is set up when operating in the CB mode. This is due to the lack of authorized personnel to operate all systems at one time in the CB mode. Eight medical personnel are required to operate the BAS (employing one CBPS) in the CB mode. At least eight nonmedical personnel are required to perform patient decontamination under medical supervision. Also, only setting up one system in the CB mode provides the BAS the ability to retain its flexibility in order to maintain its support mission of being where it is needed and when it is needed. The CBPS can be used as the treatment shelter in the conventional mode as well. When the treatment squad is operating in the split-team mode, each team will have a CBPS for use as its treatment shelter. When operating one system in the CB mode, the other system provides a replacement in the event the one in use in the CB mode is damaged beyond repair. This ensures continued HSS to the command.
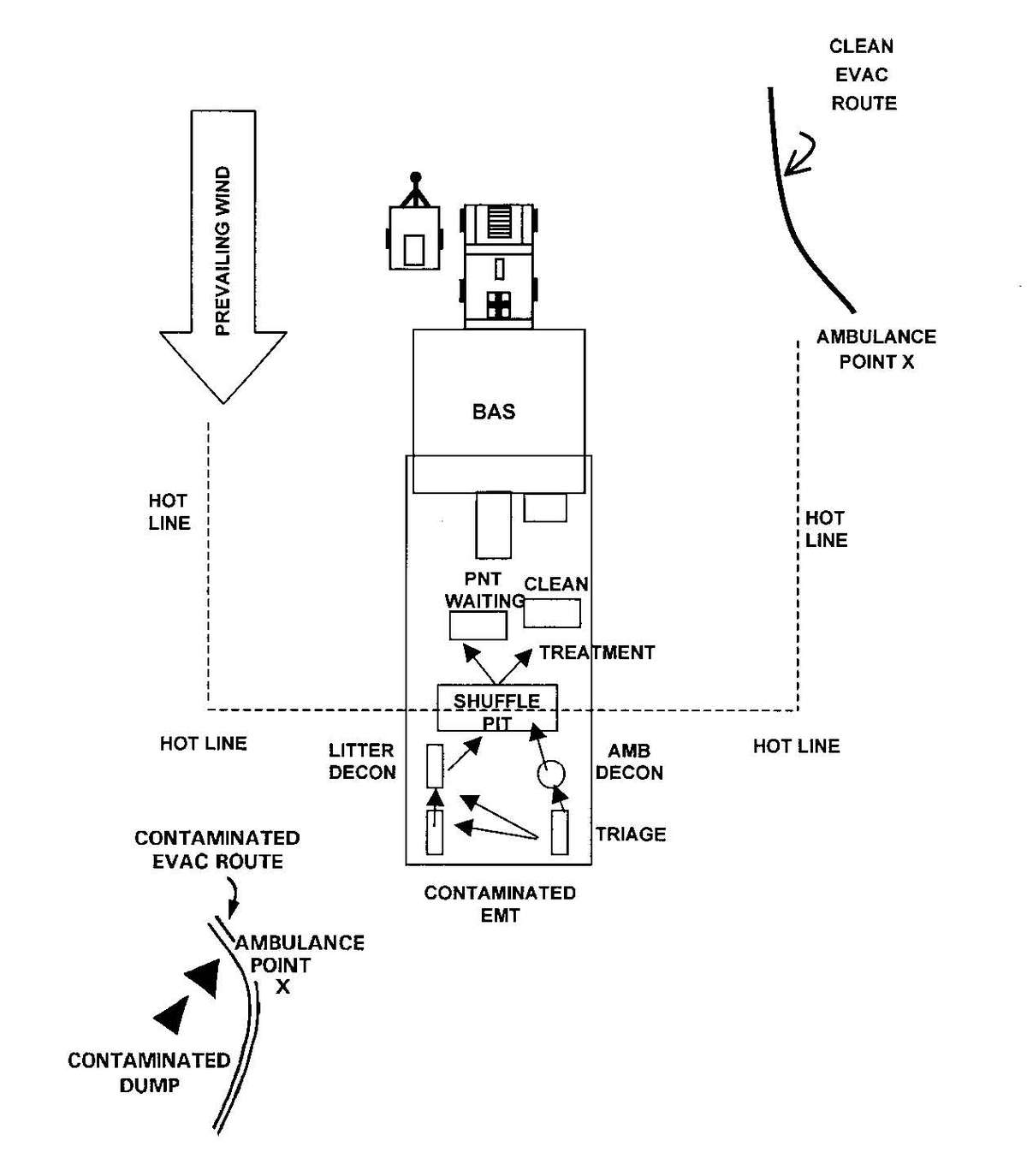
Figure F-1. Battalion aid station using the chemically biologically protected shelter.
To establish a DCS using the CBPS, set up four shelters as described in the TM. To be operational, medical supplies and equipment are set up as outlined in the unit TSOP. The four shelters are complexed as shown in Figure F-2. With four CBPS systems set up and operational, a total of 1,200 square feet of work area is available. The contaminated triage, decontamination, and contaminated treatment areas are separated from the clean treatment/waiting area by a hot line with a shuffle pit. Overhead covering is provided as described for the BAS. Patients are admitted through the EMT litter or ambulatory air lock. Patients are released through the patient holding air locks. This aids in controlling entry and exits; thus preventing the introduction of contamination into the systems. At least eight nonmedical personnel from supported units are required to perform patient decontamination under medical supervision at the DCS.
NOTE
In the event that the overpressure system fails on a system that is in use with entry/exit air locks, move to the available shelter with an entry/exit air lock in the same direction for use as the entry/exit until the failed system can be restored. Example 1: At the DCS the EMT system fails, move to the ATM shelter to receive patients until the EMT system has been restored. Example 2: At the DCS the patient hold system fails, move exits to the dental/lab/x-ray shelter until the patient hold system can be restored. Example 3: At the FST the postoperative system fails, use the preoperative shelter until the postoperative system can be restored. These options will allow patient care operations to continue until the failed systems can be restored.
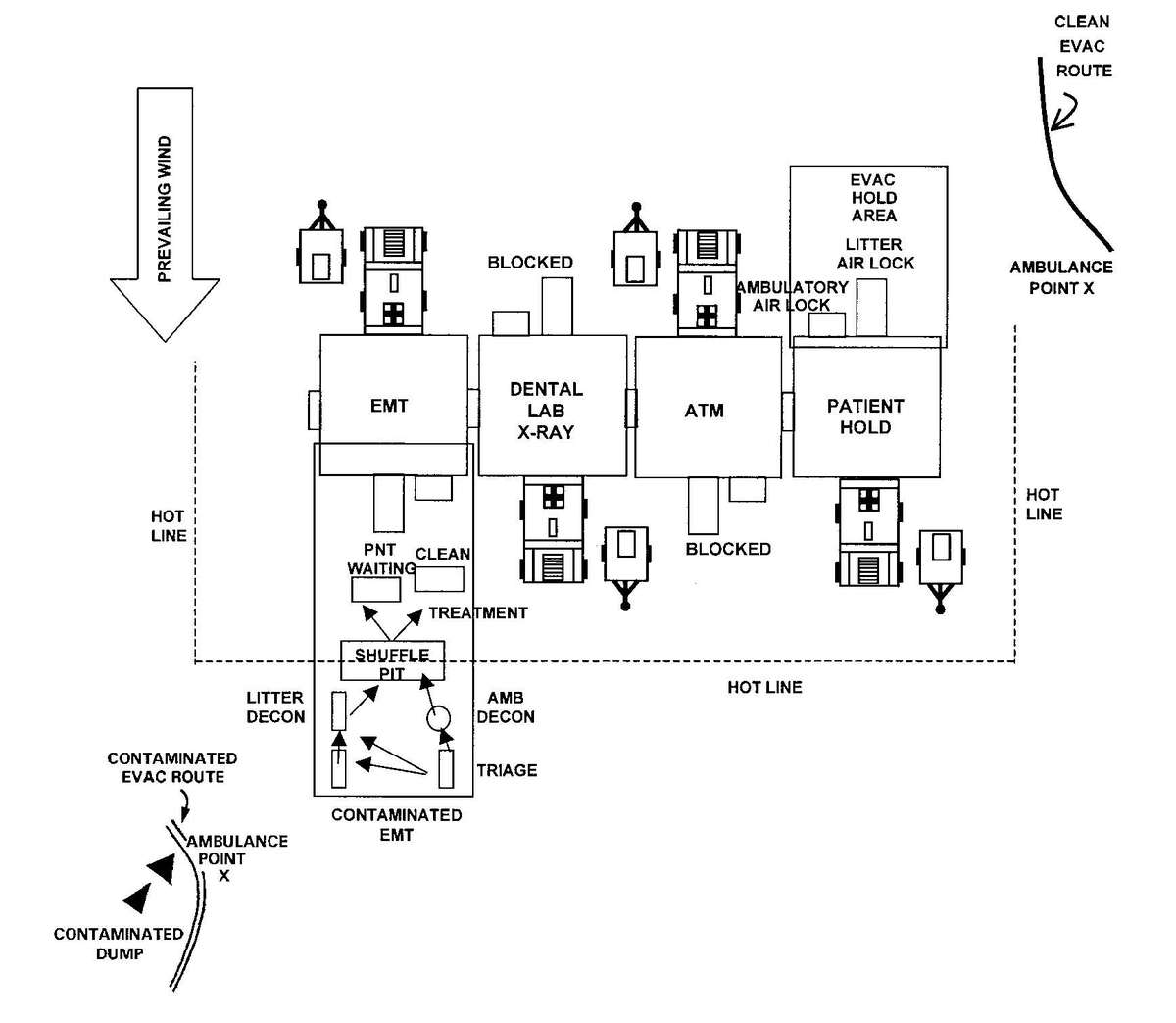
Figure F-2. Chemically biologically protected shelter configuration as a division clearing station.
To establish a FST using the CBPSs, follow the procedures for the DCS except set up three CBPSs. All equipment is set up inside the CBPS as required by your unit TSOP. With three CBPSs set up and operational, a total of 900 square feet of work area is available (Figure F-3). When the FST is forward in support of a medical company and operating in the CB mode, the FST systems are connected to the DCS of the supported medical company. Figure F-4 shows the FST and DCS connected. When operating in the CB mode with the medical company, all patients are received through the EMT air lock of the DCS. The patients are triaged in the DCS and, based upon their injuries, they are routed to the DCS treatment area or to the FST for surgical care. Patients released from the FST for evacuation are placed in a PPW and processed through the litter air lock in the FST recovery section. Patient decontamination is performed at the PDS operated by the DCS. The FST cannot operate in a CB environment without being complexed with the DCS. They do not have any patient decontamination capabilities.
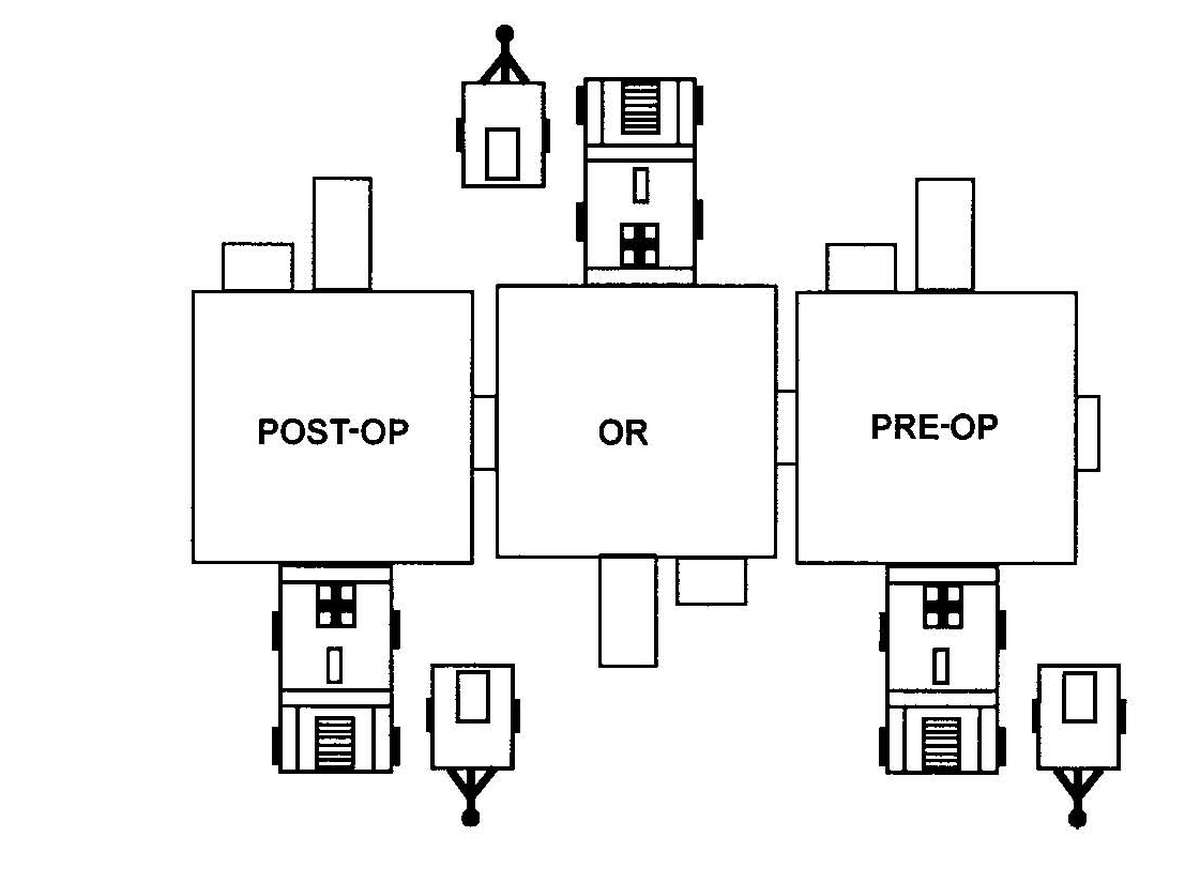
Figure F-3. Forward surgical team configuration for operations in conventional mode.
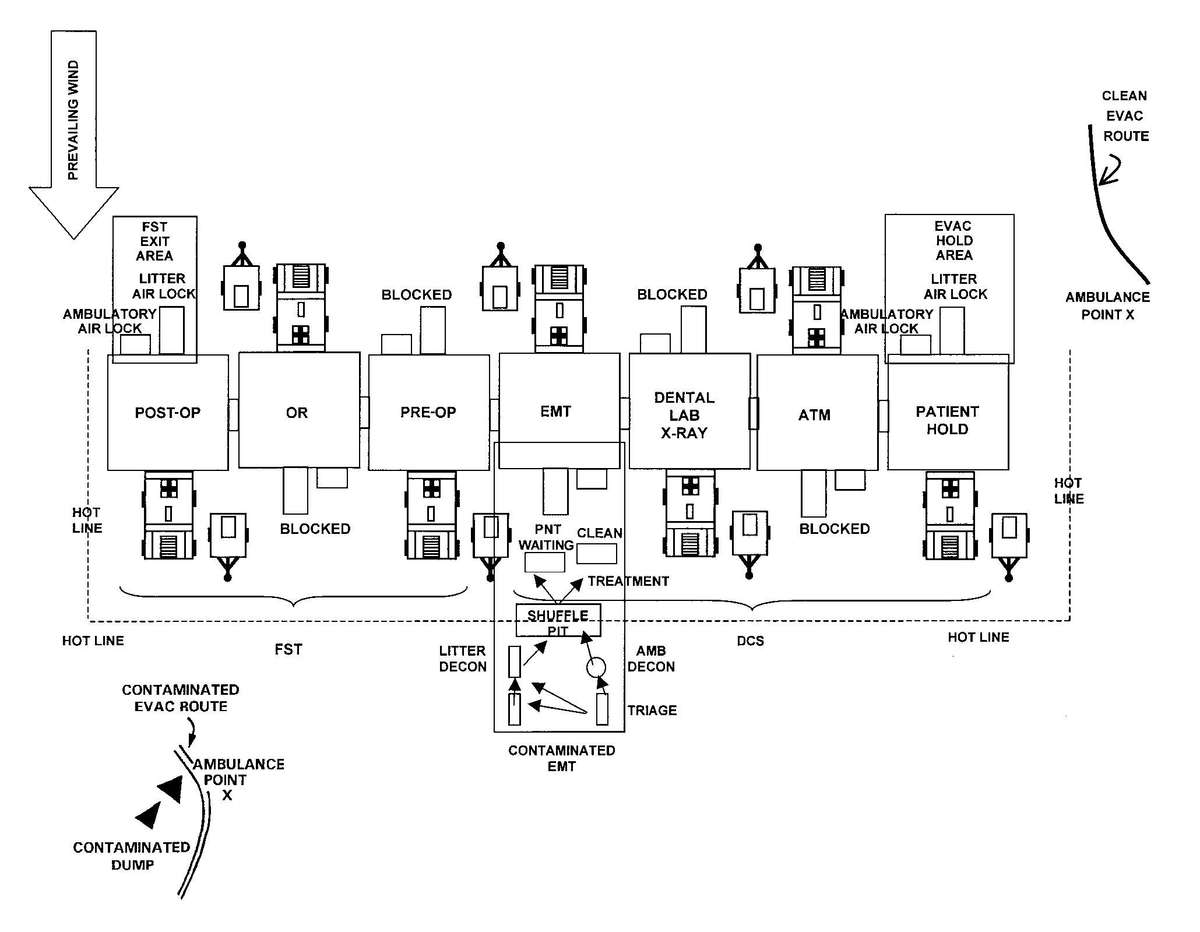
Figure F-4. Forward surgical team and division clearing station configuration for operations in a nuclear, biological, chemical environment.
a. When the threat of NBC action is anticipated in the AO, the CP DEPMEDS components must be set up as the hospital is being established. The system cannot be set up in a hospital that has already been established; to do so requires the hospital to be closed, all TEMPERs be struck, and erected with the M28 liners installed during the erection process. To establish CPS in a DEPMEDS-equipped hospital, follow the procedures as described in TM 10-5410-283-14&P. Training Circular 8-13 provides instructions on establishing a US Army DEPMEDS-equipped hospital (without CPS). Figure F-5 presents one layout of the DEPMEDS-equipped patient care area of a MF2K CSH HUB employing the CP DEPMEDS with an internal water supply system. Figure F-6 presents a layout of the patient care area of the DEPMEDS-equipped portion of an 84-bed MRI hospital. Figure F-7 presents a layout of the patient care area of the DEPMEDS-equipped portion of a 164-bed MRI hospital.
b. When employing CP DEPMEDS, provisions for waste disposal and protected water and food supplies within the system are established. Additionally, Class VIII supplies must be protected from contamination. Supplies not in use or needed in the protected operational areas are stored in medical chests, shipping containers, or wrapped in layers of plastic that are inside covered areas, such as closed MILVANs or tents. When contamination is present, only open these storage areas for operational area emergency resupply. Use plastic sheeting or other leak-proof material to provide an additional barrier between the supplies and the contamination. Wrap supplies in plastic or other barrier material for movement from the storage area to the resupply air lock of the CP DEPMEDS.
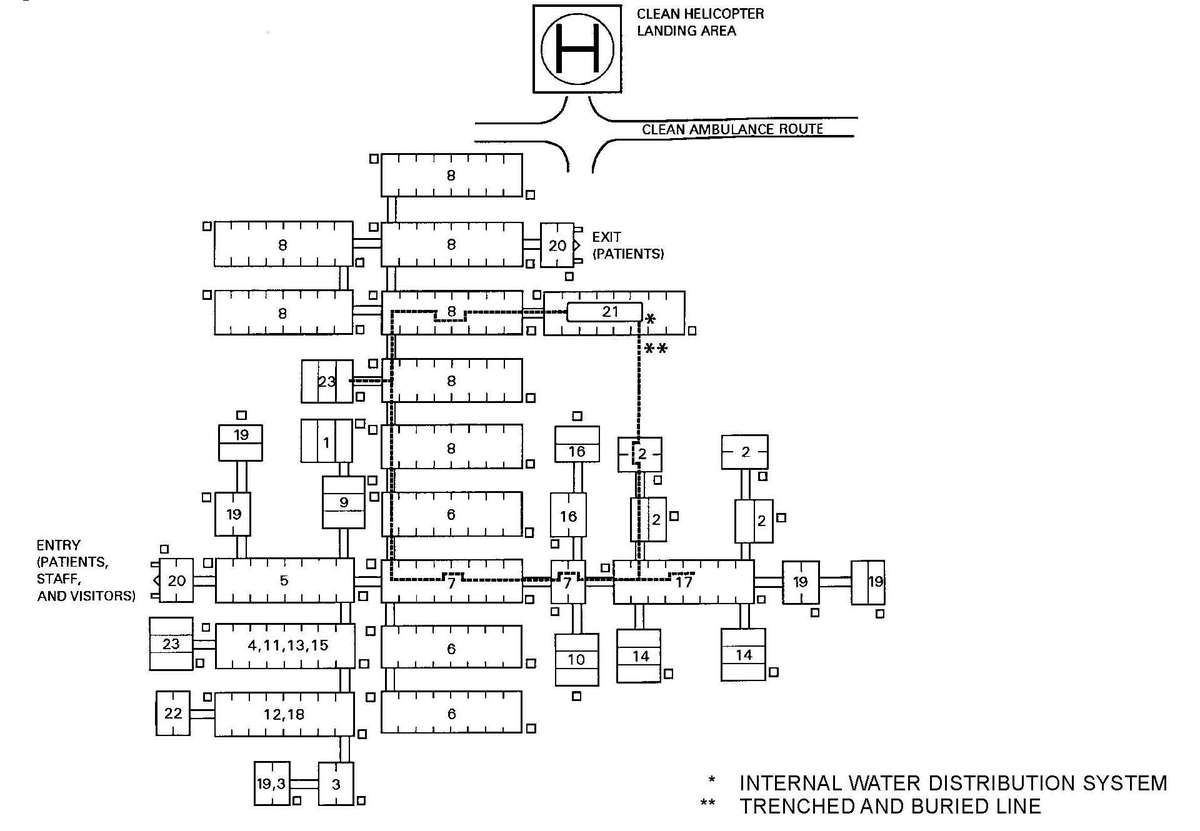
Figure F-5. Sample layout of a medical force 2000 combat support hospital unit base employing chemically protected deployable medical system.
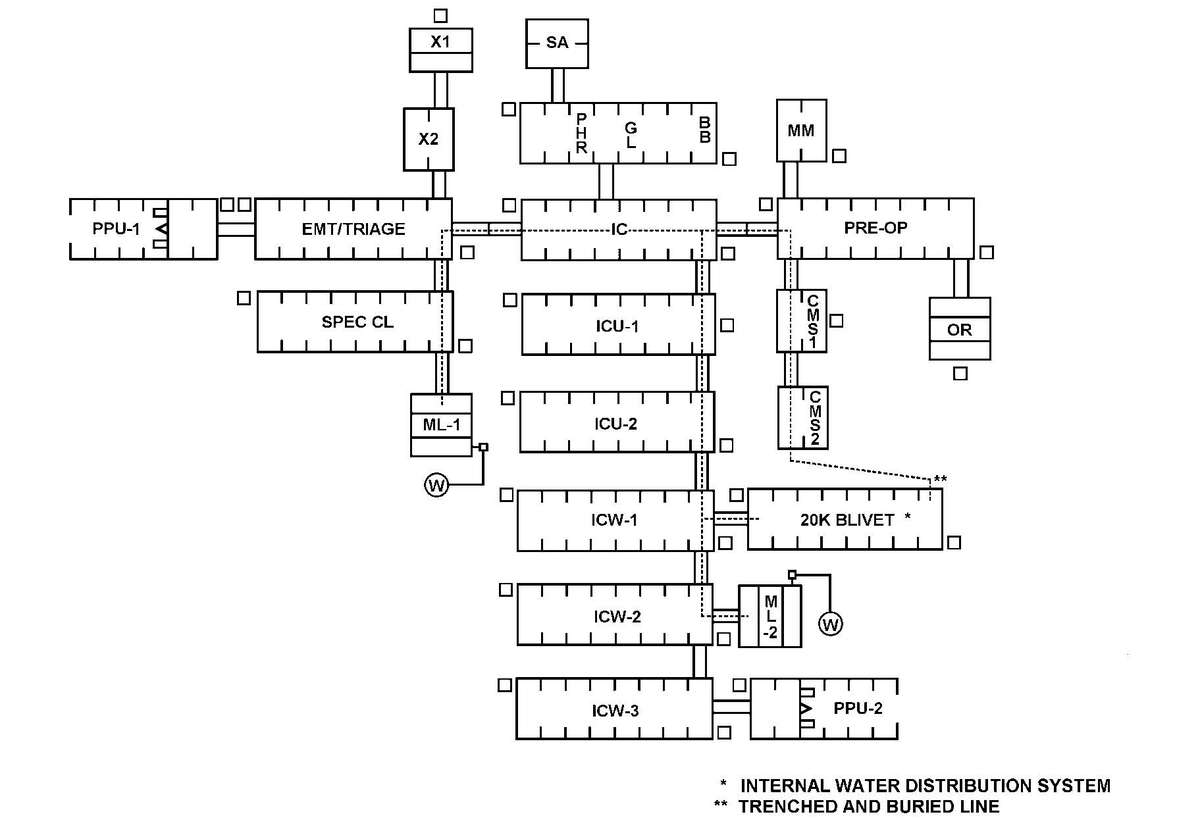
Figure F-6. Sample layout of an 84-bed medical reengineering initiative hospital employing chemically protected deployable medical system.
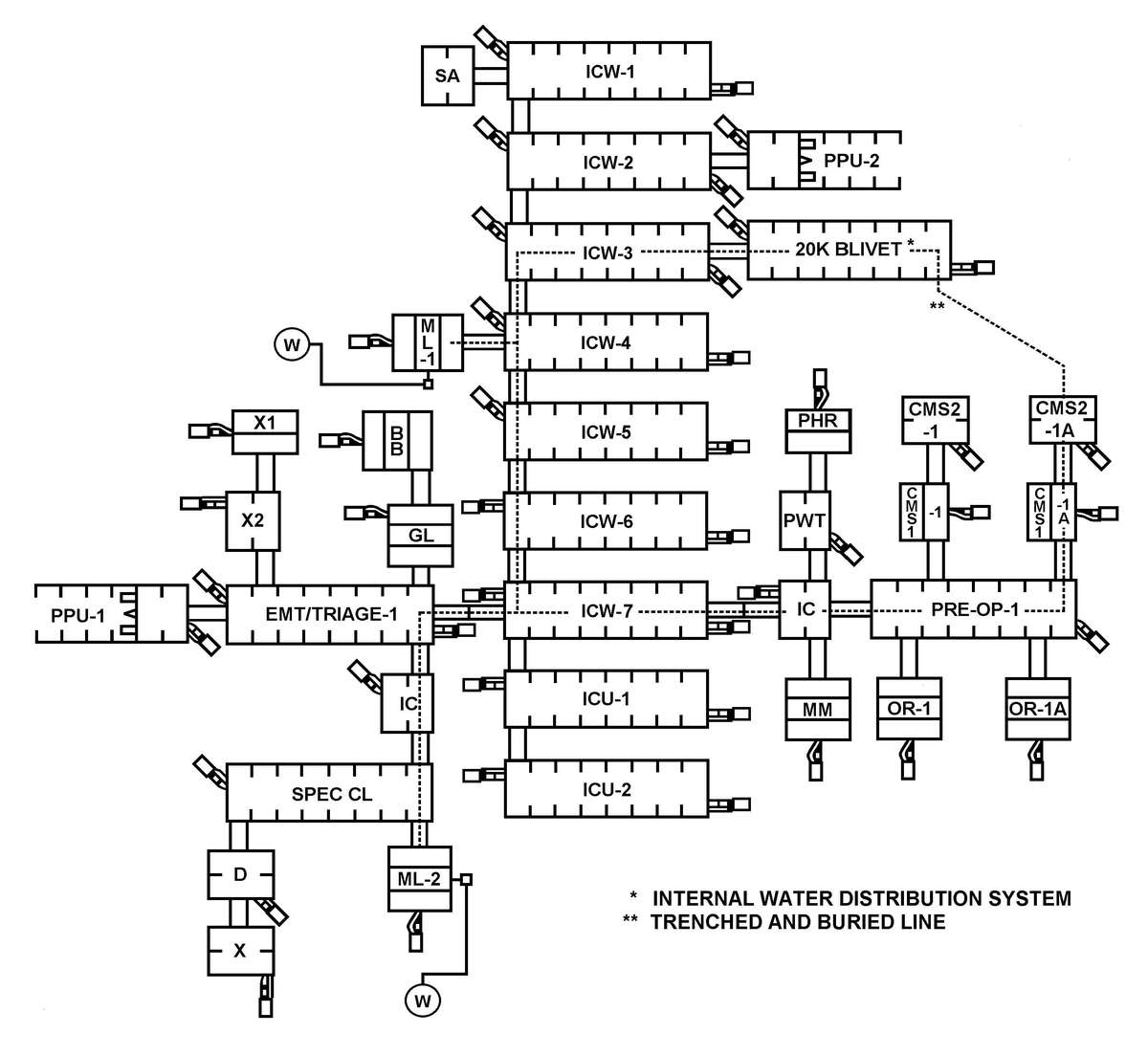
Figure F-7. Sample layout of a 164-bed medical reengineering hospital employing chemically protected deployable medical system.
To chemically/biologically protect the ISO shelters, seal all seams and openings of the ISO to prevent the entry of CB agents. The seals connecting the various sides and floor of the shelter may be a CB protected material; thus providing a seal to the shelter. When the seals are not of a CB protected material, the seams [F-12] must be taped to provide a CB protected barrier over the soft seals. Any openings not being used for introduction of support power lines, water lines or waste water lines must be sealed to prevent entry of CB agents. All access panels must be securely closed to prevent entry of vapors.
The vestibules connect TEMPERs to TEMPERs, ISOs to ISOs, and ISOs and TEMPERs. To harden the vestibules, install the CB liners inside and fasten the ends to the liners of the TEMPER or to the doors of the ISOs. Vestibule liner connectors are provided for use at the entry of each ISO.
a. The FDECU is chemically/biologically protected. The system can be operated without the CB filters. When required to operate in the CB mode, the fresh air intake on the FDECU is closed and the CB filter blower is turned on drawing fresh air through the filters to support the FDECU and to provide clean air for the CPS. Additionally, recirculation filters are placed within the shelter system to remove any agent that may have entered through any of the entry/exit areas or through breaches in the shelter system.
b. When heaters are required, they must be chemically/biologically protected to prevent entry of contamination. The CB filter units are connected to the fresh air intake side of the heater and the heated air discharge side of the heater is connected to the air supply of the TEMPER/ISO.
The M20 is used to establish a CPS within a room of opportunity, or inside a tent; however, the available space will be limited by tent poles and other components of the tent. Currently this system only provides ambient temperature air. See the TM and manufacturer's publication provided with the system and system components for details.
NOTE
The M20 does not have a litter air lock. Only staff or ambulatory patients can enter. See the TM provided with the system for setup procedures.
Patients admitted into the MTF must be contamination free. Therefore, a casualty decontamination area must be established near the MTF. The casualty decontamination area should be provided with an overhead cover as described for the CBPS system, except that it does not overlap the entry to the hospital. Also, [F-13] consideration must be given to the location of other operations at the hospital site when establishing the casualty decontamination area. However, the area must be close enough to the entry/exit of the CPS to protect the patients from the environment and reduce their exposure to recontamination. Keep in mind that under NBC conditions personnel outside of the CPS are at MOPP Level 4 (except decontaminated patients; they have their mask on), thus increasing the stress load and reducing their overall performance capabilities. The entry/exit area must have overhead cover to protect patients awaiting access to the CPS. See Appendix I for setting up a casualty decontamination area and for decontamination procedures.
These operations, entry, and exit guidelines may be used to prepare a unit SOP for the operation of CPS systems in your unit.
a. When using these guidelines, the following should be considered:
b. Information on setting up, striking, and operating the CPS is contained in the equipment publications. Where applicable, special procedures are provided in these publications for setting up in both clean and CB vapor hazard areas. However, the CP DEPMEDS is NOT set up in a CB vapor hazard area. The commander will determine which procedures to use.
c. During operations, periodic checks are made of the atmosphere within the shelter. These checks are made by using available chemical agent detection equipment and material to determine if chemical agent penetration has occurred. Should chemical agent penetration occur, all personnel must mask; then ensure that patients are protected until the agent has been purged from the shelter.
a. Normally, the MTF will not operate in a CB vapor hazard environment. However, if the MTF must remain in an area on a temporary basis and liquid agent contamination is present, the immediate area around the entrance must be decontaminated.
b. To decontaminate the area around the entrance, use one or more of the following methods:
All personnel (staff and patients) must be decontaminated before they are permitted entry into the CPS.
WARNINGS
1. ALWAYS PURGE THE AIR LOCK BEFORE OPENING THE INNER DOOR, IF THE OUTER DOOR HAS BEEN OPENED.
2. WHEN OPERATING IN A TOXIC ENVIRONMENT, NEVER OPEN THE OUTER AND INNER DOORS OF THE AIR LOCKS AT THE SAME TIME.
a. Ambulatory Personnel.
(1) Entry procedures.
(a) Ambulatory patients and others remove their MOPP (except their mask), BDUs, and boots outside the air lock. This procedure reduces the amount of possible contamination entering the air lock.
(b) A check is made to ensure that the ambulatory air lock is empty and the inner door is closed.
(c) The individual enters the air lock and closes the outer door.
(d) The air lock is purged for 3 minutes. At the end of the purge cycle, the individual checks for contamination. If contaminated, the individual must return to the outside and decontaminate his skin; then return to the air lock and repeat the purge cycle and contamination check. If no contamination is detected, the protective mask is removed and placed in a plastic bag. The plastic bag is sealed and labeled. The individual opens the inner air lock door and enters the CPS; the plastic bag is carried into the shelter with the individual.
(2) Exit procedures.
(a) A check is made to ensure that the ambulatory air lock is empty and the outer door is closed.
(b) The individual enters the air lock and closes the inner door.
(c) The individual puts on his protective mask; then exits through the outer door.
(d) The individual puts on his BDU and boots then assumes the established MOPP level before departing the immediate area of the exit door.
WARNING
DO NOT OPEN THE OUTER DOOR UNTIL THE PROTECTIVE MASK HAS BEEN PUT ON.
NOTES
1. Ambulatory patients that enter the CBPS become litter patients and are placed in PPW when released because the MTF does not have replacement MOPP ensembles for patient issue.
2. Exits must be spaced so that at least a 3 minute purge of the air lock is accomplished before the inside door is opened. Only open the doors long enough to permit passage.
b. Litter Patients.
(1) Entry procedures.
(a) An outside aidman notifies an inside aidman that a litter patient is ready for admission.
(b) The inside aidman ensures that the inner litter air lock door is closed. The outside aidmen open the outer air lock door and place the litter on the litter rails; they push the patient into the air lock headfirst; then they close the outer door. After a purge time of 3 minutes, an aidman inside the CPS opens the inner door and checks the patient to ensure that he is contamination free. The patient is checked by placing the CAM nozzle near absorptive surfaces, such as the patient's hair. If no contamination is found, the aidman removes the patient's mask and places it in a plastic bag. The inside aidmen remove the patient from the air lock and position him on treatment litter stands, or move him to the treatment area as directed by supervisory personnel.
(c) Patients received at the treatment facility in the PPW are checked for contamination; if they are contamination free, they may be processed through the litter air lock in the PPW. The inside aidmen ensure that the inner litter air lock door is closed. The outside aidmen open the outer air lock door and place the litter on the litter rails and push the patient into the litter air lock headfirst, then close the outer door. Purge the air lock for 3 minutes. After the purge time, an aidman inside of the CPS opens the inner air lock door and uses the CAM to check the patient to ensure that he is free of contamination. If no contamination is found, the inside aidmen remove the patient from the air lock. (If the patient is wearing a protective mask, the mask is removed and placed in a plastic bag before the patient is moved from the air lock.) As the patient is removed from the air lock, the PPW is opened and rolled inside out so that any desorbing vapors are adsorbed by the charcoal layer. The inside aidmen remove the patient from the air lock and position him on litter stands. The patient is transferred to a clean litter; then moved to the treatment area as directed by supervisory personnel. The receiving litter and PPW is returned to the outside; dispose of the PPW in the contaminated waste dump. Decontaminate the litter and return it to the litter pool.
NOTE
Should contamination be found when monitoring the air lock in (b) or (c) above, repeat the purge cycle, then retest for contamination. All vapor hazards must be eliminated before the patient is moved into the CPS. Repeating the purge cycle may NOT be possible if the patient is in need of immediate lifesaving care. The patient may have to be returned to the outside treatment area for immediate care.
(2) Exit procedures.
(a) The litter patient is placed in a PPW. A battery operated blower unit with a CB filter may be attached to the PPW to provide fresh air to the patient; thus reducing the heat load on the patient and the carbon dioxide buildup inside the PPW.
(b) An inside aidman notifies an outside aidman that the patient is ready to exit the shelter. An outside aidman ensures that the outer air lock door is closed. The patient is placed in the litter air lock feet first. The inner air lock door is closed. The outside aidmen open the outer door and remove the patient.
(c) Hospital staff, visitors, or ambulatory patients exit through the ambulatory air lock. Before entering the air lock, each individual must ensure that the outer air lock door is closed. The individual enters the air lock and closes the inner door; puts on his protective mask and exits through the outer door. The individual puts on his BDU and boots, and then assumes the established MOPP level before departing the immediate area of the exit door.
WARNING
DO NOT OPEN THE OUTER DOOR UNTIL THE INNER DOOR HAS BEEN CLOSED.
NOTE
Exits must be spaced at least 3 minutes apart to allow for a complete purge cycle of the air lock.
Resupply of protected areas is accomplished by placing contamination-free supplies or equipment on a litter and passing it through the litter air lock, or processing it through the supply air lock. The litter air lock must be purged for 3 minutes. The supplies must be checked for contamination before they are removed and placed within the CPS. The supply air lock must be purged for the stated time as outlined in the supporting technical manual; usually 45 minutes. Again the supplies must be checked for contamination before they are removed and placed within the CPS.
a. Patient decontamination presents special problems for units and HSS personnel. Nuclear, biological, and chemical contaminated patients create increased hazards to rescuers and HSS personnel; thus, causing delays in providing essential first aid and medical treatment for injuries from sources other than the exposure to NBC weapons/agents. Casualty decontamination procedures are performed by each individual, as buddy aid, or at a unit decontamination station prior to the arrival of medical personnel. See FM 3-5 for procedures on individual, buddy aid, and unit decontamination. Patient decontamination procedures are normally performed at an MTF under medical supervision. Patient decontamination stations may be established (collocated) at central unit decontamination faculties, if medical support is available. However, augmentation medical support must be requested to provide patient care and supervise the patient decontamination process. Because, when the unit is undergoing decontamination operations, organic medical personnel must also decontaminate their equipment and personnel. Therefore, they are not available to provide medical support for operating the patient decontamination station that is collocated with the central unit decontamination facility.
b. The term "decontamination" as used herein means the removal or neutralization of radioactive particles, BW agents, and CW agents to levels low enough that patients may be treated without contaminating the MTF and without posing health risks to unprotected medical providers. "Decontamination" does not imply absolute removal of contaminants.
NOTE
The decontamination procedures described below are for NBC contaminated patients. These procedures may also be used for most TIM contaminated patients. However, soap and water will suffice for most TIMs; but some TIMs react with water. For those TIMs another material must be used to decontaminate patients. For detailed information on decontamination of TIM contaminated patients, see FM 8-500.
c. Physical removal of contaminants is the primary method of decontamination. Physical removal does not require vigorous scrubbing; in fact, vigorous scrubbing can force some agents deeper into the skin; thus, increasing the agent effect rather than reducing its effects. The use of a M291 skin decontaminating kit (SDK) neutralizes/reduces the effects of an agent, but physical removal is of utmost importance. When a SDK is not available, the use of soap and water should be considered as the next best method. However, the use of soap and water requires large amounts of water that may not be available because the soap must be rinsed from the skin to reduce skin irritation from the soap. An alternate skin decontaminant is a hypochlorite solution; but it should only be used when SDKs and/or sufficient quantities of water are not available. Use a 0.5 percent hypochlorite solution on the protective mask and skin. A 5 percent hypochlorite solution can be used on the mask hood, gloves, and other outer garments.
CAUTION
Do not use the 5 percent solution on the skin; it can cause severe skin irritation.
Decontamination must begin at the platoon and company level with the individual soldier, prior to the arrival of medical personnel. The soldier himself or members of his team must perform immediate decontamination. When the casualty's condition and the mission permits, they may go through a MOPP gear exchange at their unit before evacuation (see FM 3-5). Performing a MOPP gear exchange at the unit before evacuation will reduce the amount of contamination that can be transferred to the MEDEVAC vehicle. However, the MOPP gear exchange must not cause further injury to the casualty. First aid for CW agent must be administered; such as administering nerve agent antidotes (such as nerve agent antidotes and convulsant antidote for nerve agent [CANA]), as required. Enter the time and type of contamination on a field expedient NBC casualty card (Figure G-1). Use the CAM, M8 chemical agent detector paper, or M9 tape to determine the type of chemical contamination. Use a radiation detection meter/device to determine the level of radioactive contamination, if required. Currently, there are no BW agent detectors that can be used to check patients for BW agent contamination. Therefore, all patients suspected of being contaminated with a BW agent must be decontaminated. When medical personnel arrive, they should enter the time and type of contamination and number of antidote injections that were administered as first aid on the Department of Defense (DD) Form 1380 (Field Medical Card [FMC]).
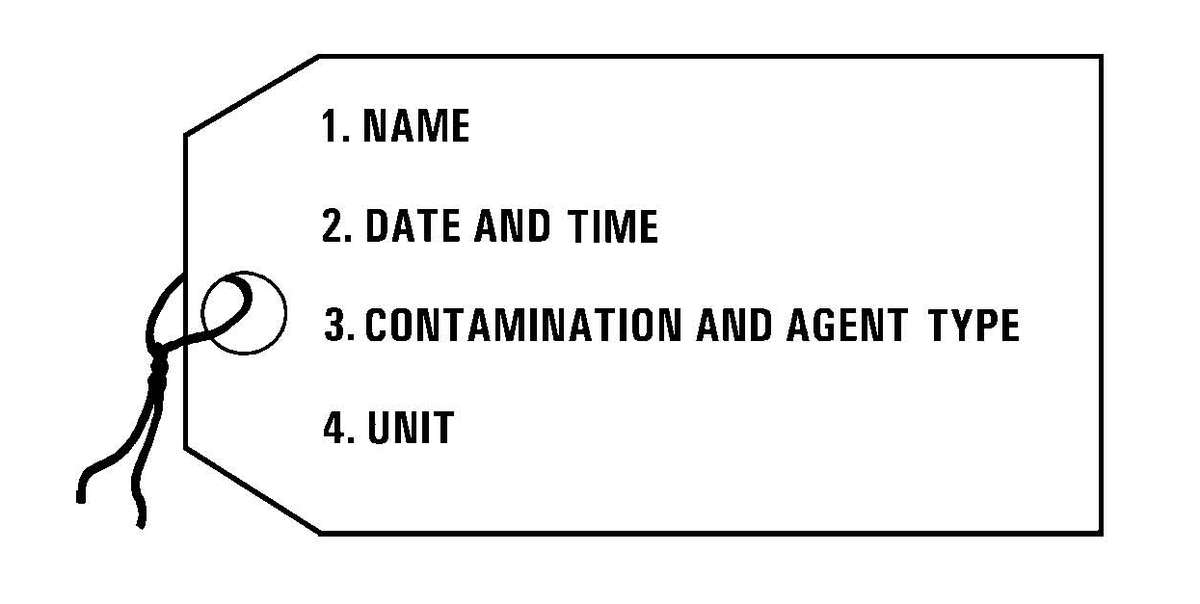
Figure G-1. Field expedient nuclear, biological, and chemical patient card.
a. Collocating patient and thorough decontamination operations in the BSA may provide several advantages (Figure G-2). It—
b. An identified disadvantage is the increased size of the site and the requirement for medical support augmentation (a treatment squad from another organization with required patient decontamination and treatment MESs) to operate the PDS.
NOTE
Organic medical personnel must not be used to perform the HSS mission at the collocated site. They must go through the decontamination process with their unit.
c. These operations do not require that both patient decontamination and unit thorough decontamination be executed simultaneously. The PDS can be running while the thorough decontamination site is being prepared. Patient decontamination cannot be delayed since patients may be suffering life-threatening injuries as well as exposure to NBC agents. Therefore, the PDS must be established and operational before the first patients arrive. The wind direction must be common to both sites.
d. The decontamination platoon leader is responsible for establishing the combined decontamination site. The medical unit commander/surgeon coordinates with the decontamination platoon leader for the location of the patient receiving, PDS, and MTF. The lowest level at which this operation will usually be planned is brigade. This operation requires extensive planning and must involve the brigade chemical officer, brigade S4, and the medical company commander/brigade surgeon. Decontamination support for special operation forces, other unique operational organizations, or for nonlinear operations may require execution at a lower level. The supporting medical personnel operate the PDS. Nonmedical personnel perform patient decontamination procedures under medical supervision. Patient decontamination procedures are described below.
NOTE
Patient decontamination differs from thorough decontamination in that the patients' medical status must be monitored and medical treatment must be provided during the decontamination process.
e. Although a PDS may be collocated with thorough decontamination, a PDS must be operational at Levels I, II, III, and IV MTFs. Contaminated patients may present directly to the MTF for care, or patients previously decontaminated may become contaminated en route. Therefore, all patients arriving at an MTF must be checked for contamination. If contaminated, they must be decontaminated before they are admitted to the MTF.
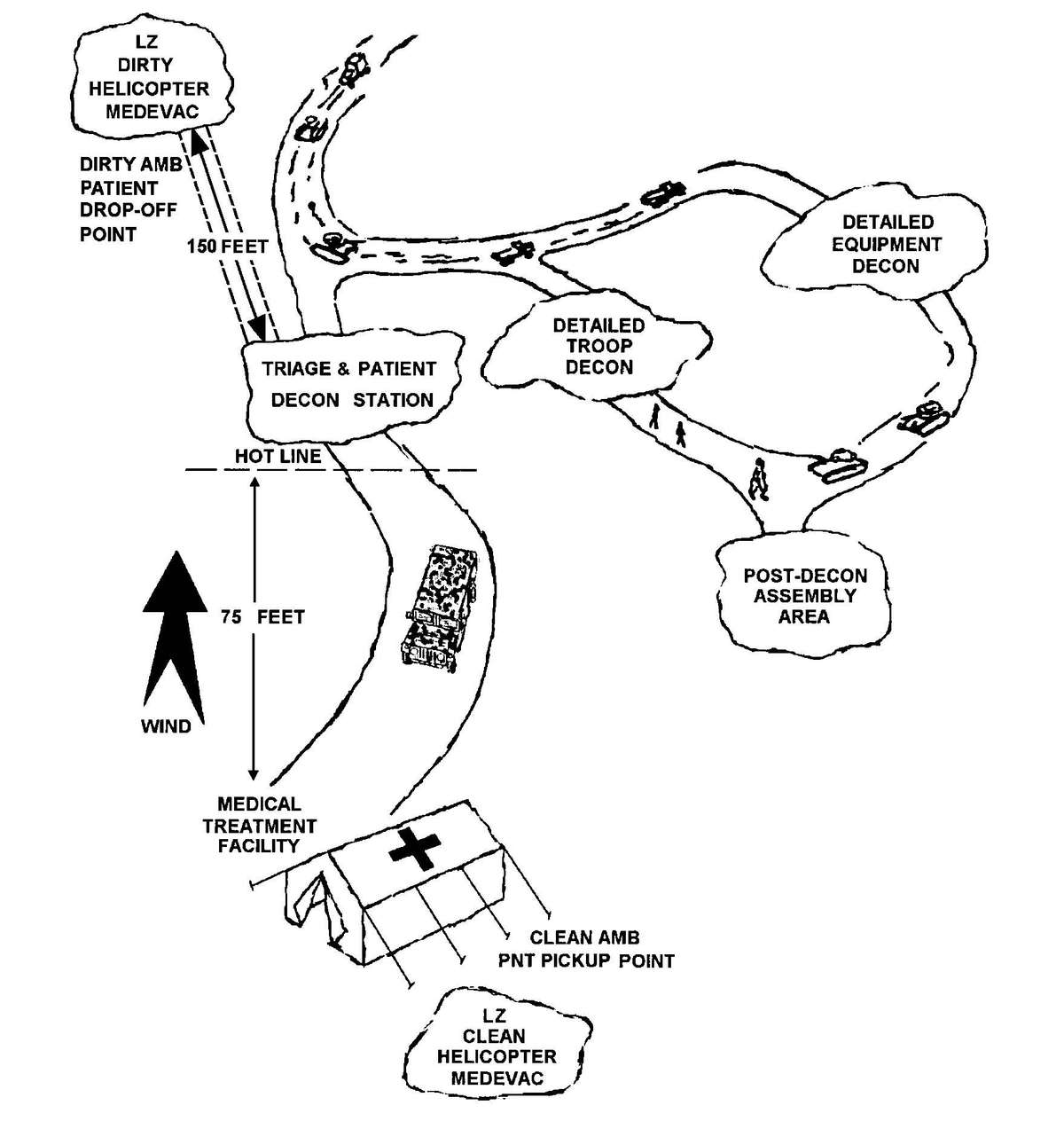
Figure G-2. Thorough decontamination site collocated with patient decontamination station, without collective protection shelter.
a. When battle conditions prevent patient decontamination procedures forward or the patient is contaminated en route, the patient may have to be decontaminated at the BAS. Contaminated patients arriving at the BAS must be decontaminated before admission into the clean treatment area.
b. Patient decontamination is performed by eight nonmedical personnel from the supported unit at the BAS. The patient decontamination personnel operate as two-man teams to perform the patient decontamination procedures. The patient decontamination teams operate under the supervision of medical personnel to ensure that no further injury is caused to the patient by the decontamination process. Each team receives a patient from the triage point and performs both clothing removal and skin decontamination procedures. The team requires assistance from another team to perform litter changes; see details below.
The medical company clearing station may receive patients from the BAS or directly from other areas who have not been decontaminated. The clearing station must also have a patient decontamination area. As with the BAS, the clearing station must have a minimum of eight nonmedical personnel from the supported units to perform patient decontamination. Procedures for patient decontamination at the clearing station are the same as for the BAS.
To the maximum extent possible, hospitals are located away from tactical or logistical targets. Contaminated patients will arrive from forward MTFs and units located within the geographical area of the hospital. Patient decontamination is done by at least 20 nonmedical personnel from units located in the geographical area/base cluster of the hospital. Procedures for patient decontamination at the hospital are the same as for the BAS. However, several patient decontamination stations can be operated simultaneously at the hospital patient decontamination site. Further, all patients arriving at the hospital will be decontaminated and receive full treatment within the capabilities of the hospital.
An alternative patient decontamination agent is a hypochlorite solution; however, the hypochlorite solution must be prepared. Two concentrations of the hypochlorite solution are required. A 5 percent hypochlorite solution to decontaminate gloves, aprons, litters, cutting devices, the patient's mask hood, and other nonskin contact areas. The patient's mask, skin, splints, and tourniquets and their wounds are irrigated using a 0.5 (½) percent hypochlorite solution. To prepare the solutions, use calcium hypochlorite (HTH) granules (supplied in 6-ounce jars in the chemical agent patient treatment and chemical agent patient decontamination MES), bulk HTH, or sodium hypochlorite (household bleach). Prepare the required solutions as shown in Table G-1 below.
Table G-1. Preparation of Hypochlorite Solutions for Patient Decontamination
| HTH OUNCES | HTH MRE SPOONFULS | HOUSEHOLD BLEACH | PERCENT IN 5 GALLONS OF WATER |
|---|---|---|---|
| 6 | [*]5 | 2 QUARTS | 0.5 |
| 48 | 40 | [**] | 5.0 |
[*] THESE MEASUREMENTS ARE USED WHEN BULK HTH IS USED. TO MEASURE THIS PREPARATION, USE THE PLASTIC SPOON SUPPLIED WITH YOUR MEAL, READY-TO-EAT (MRE). THE AMOUNT OF HYPOCHLORITE TO BE USED IS A HEAPING SPOONFUL (THAT IS, ALL THAT THE SPOON WILL HOLD). DO NOT SHAKE ANY GRANULES OFF OF THE SPOON BEFORE ADDING TO THE WATER. | |||
[**] DO NOT DILUTE IN WATER; HOUSEHOLD BLEACH IS 5 TO 6.25 PERCENT SOLUTION; IT IS USED FULL STRENGTH FOR 5 PERCENT APPLICATIONS. | |||
CAUTIONS
1. Do not use the 5 percent hypochlorite solution on the patient's skin. The 5 percent solution can burn the skin.
2. Only wipe the skin when applying the 0.5 percent hypochlorite solution. Vigorous scrubbing may force the agent into the skin.
On the NBC battlefield, two classifications of patients will be encountered—contaminated and uncontaminated. Those contaminated may suffer from the effects of an NBC agent, of a conventional wound, or both. Some may suffer combat stress or heat injuries induced by the stress of NBC conditions and extended time spent in MOPP Level 4. It is important to follow proper decontamination procedures to limit the spread of contamination to others and equipment. The most important decontamination is performed at the site of contamination. Decontamination at a later time may be too late to prevent injury to the individual, especially when exposed to vesicants. All agents should be promptly removed from the skin.
This appendix only describes patient decontamination procedures. For NBC treatment procedures, refer to FM 4-02.283, FM 8-284, and FM 8-285.
Before contaminated patients receive medical treatment in the clean treatment area, they must be decontaminated. Place the cutting device in a container of 5 percent hypochlorite solution between each use. Each decontamination team member decontaminates his gloves and apron with the 5 percent hypochlorite solution frequently to prevent spreading any contamination to patient's skin. Decontaminate the patient's skin, bandages, wounds, mask, identification tags with chain, and splints with a 0.5 percent hypochlorite solution. The litter patient is decontaminated and undressed as follows:
NOTE
Litter patients requiring EMT or ATM in the clean area of the MTF will be completely decontaminated. A patient not requiring clean EMT or ATM at the MTF, but requiring further evacuation (for example: a stable patient with a partial amputation of a lower extremity) should only have his wound area and MOPP spot decontaminated to remove any gross contamination. The patient should be evacuated in his MOPP.
a. Step 1. Physically remove gross contamination. Use any stiff material (stick, cardboard, plastic strip, metal banding strap) to physically remove gross contamination from the patient's MOPP ensemble. Much of the CW agent contamination can be removed through physical means.
b. Step 2. Decontaminate the patient's mask and hood. The patient has been triaged and stabilized (if necessary) by the senior trauma specialist in the patient decontamination area. A two-man decontamination team moves him to the litter stands at the clothing removal station.
(1) Decontaminate the mask and hood. Use the SDK, or use a 5 percent hypochlorite solution or household bleach to sponge down the front, sides, and top of the mask hood. Decontaminate spots with the SDK or the 5 percent hypochlorite solution.
(2) Remove hood. Remove the hood by cutting the hood. Before cutting the hood, dip the cutting device in a 5 percent hypochlorite solution. For the M17-series mask, cut the neck cord and the small string under the voicemitter. Release or cut the hood shoulder straps and unzip the hood zipper. Cut the hood, close to the filter inlet cover and eye-lens outsert, upward to the top of the eye-lens outsert, and across the forehead to the outer edge of the other eye-lens outsert. Proceed downward toward the patient's shoulder, staying close to the eye-lens, then across the lower part of the voicemitter to the zipper. After dipping the cutting device in the 5 percent hypochlorite solution, cut the hood from the center of the forehead over the top of the head (see Figure G-3). Fold the left and right sides of the hood to the side of the patient's head, laying the sides of the hood on the litter. For the M40-series protective mask cut the [G-8] hood shoulder straps, then cut the quick-doff hood from the front bottom center to the chin through the elastic band under the chin. Fold the left and right sides of the hood over the shoulders away from the head.
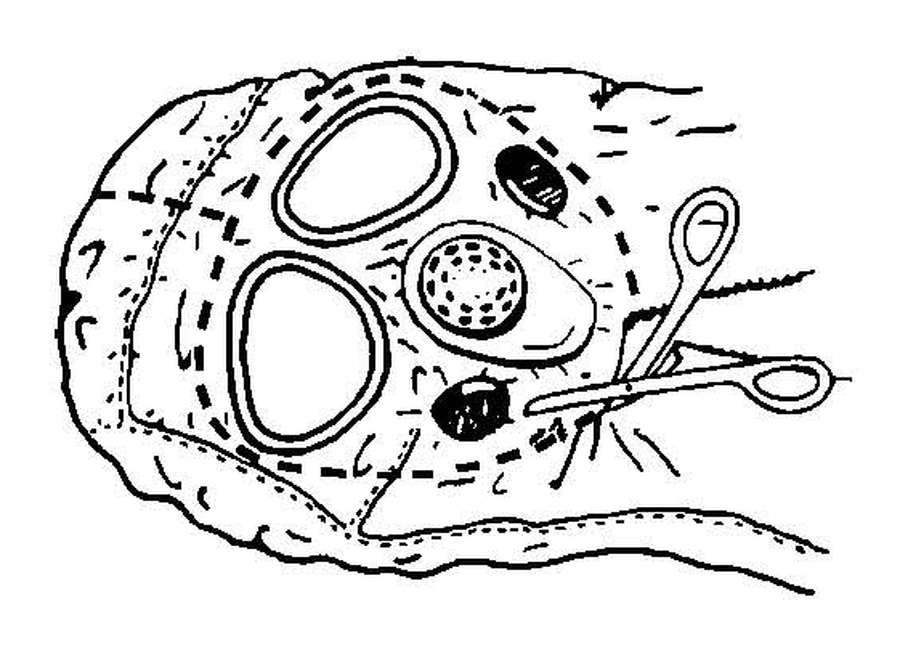
Figure G-3. Cutting the M17 protective mask hood.
(3) Decontaminate the protective mask and exposed skin. Using the SDK, soap and water, or a 0.5 percent hypochlorite solution, wipe the external parts of the mask. Cover the mask air inlet(s) with gauze or your hand to keep the mask filter dry. Continue by wiping the exposed areas of the patient's face, including the neck and behind the ears.
(4) Remove the Field Medical Card. Cut the patient's FMC tie wire, allowing the FMC to fall into a plastic bag. Seal the plastic bag and rinse the outside of the bag with a 5 percent hypochlorite solution. Place the plastic bag with the FMC under the back of the protective mask head straps. The FMC will remain with the patient.
c. Step 3. Remove gross contamination from the patient's overgarment. Remove all visible gross contamination by scraping with a stick or other device.
d. Step 4. Remove the patient's personal effects and protective overgarment.
(1) Remove patient's personal effects. Remove the patient's personal effects from his protective overgarment and BDU pockets. Place the articles in a plastic bag, label with the patient's identification, and seal the bag. If the articles are not contaminated, return them to the patient. If the articles are contaminated, place them in the contaminated holding area until they can be decontaminated, and then return them to the patient.
(2) Cut the patient's overgarment. The overgarment jacket and trousers may be cut simultaneously. Two persons may be cutting clothing at the same time. Cut around bandages, tourniquets, and splints, leaving them in place.
NOTE
A cut is a separation of material by use of a cutting device that cuts material into two pieces. EXAMPLE: Cutting the sleeve from the cuff to the jacket collar is one cut.
CAUTION
Bandages may have been applied to control severe bleeding and are treated like tourniquets. Only medical personnel remove bandages, tourniquets, and splints.
(3) Remove overgarment jacket. Make two cuts, one up each sleeve from the wrist up to the shoulder, and then through the collar (Figure G-4). Do not allow the gloves to touch the patient along the cut line. Dip the cutting device in the 5 percent hypochlorite solution before making each cut to prevent contamination of the patient's uniform or underclothing. Keep the cuts close to the inside of the arms so that most of the sleeve material can be folded outward. Unzip the jacket; roll the chest sections to the respective sides, with the inner surface outward. Continue by tucking the clothing between the arm and chest. Roll the cut sleeves away from the arms, exposing the black liner.
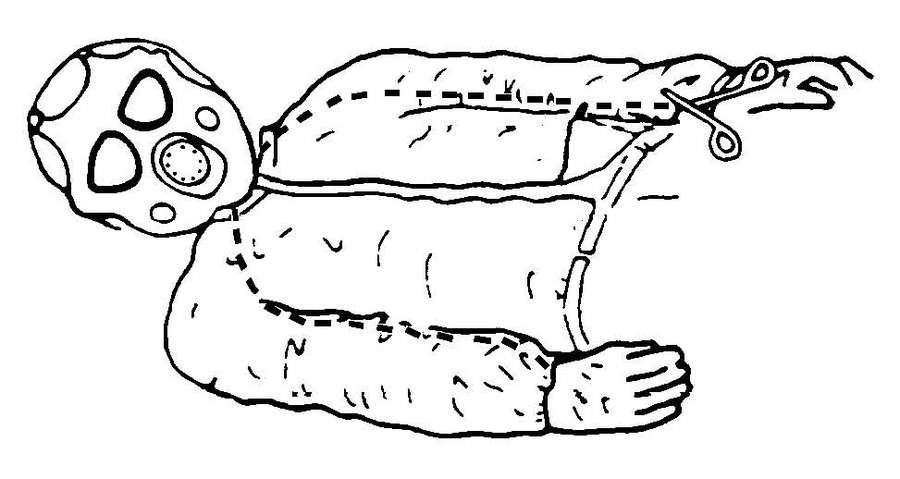
Figure G-4. Cutting the overgarment jacket.
(4) Remove overgarment trousers. Cut both trouser legs starting at the ankle as shown in Figure G-5. Keep the cuts near the inseams to the crotch. With the left leg, continue cutting to the waist, avoiding the pockets. With the right leg, cut across at the crotch to the left leg cut. Place the cutting device in the 5 percent hypochlorite solution. Fold the cut trouser halves away from the patient and allow the halves to drop to the litter with contaminated (green) side down. Roll the inner leg portion under and between the legs.
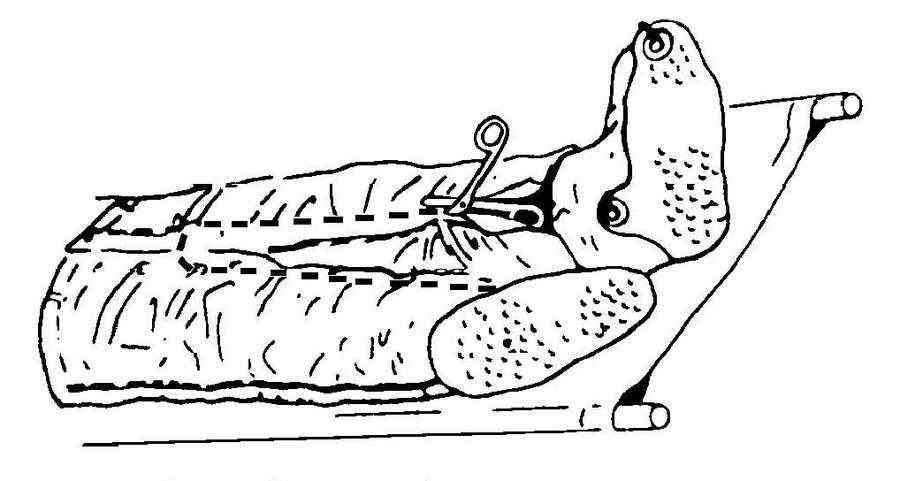
Figure G-5. Cutting the overgarment trousers.
(5) Remove outer gloves. This procedure can be done with one person on each side of the patient working simultaneously. The decontamination team will decontaminate their gloves in 5 percent hypochlorite solution. Next, lift the patient's arms up and out of the cutaway sleeves unless detrimental to the patient's condition. Grasp the fingers of the glove, roll the cuff over the fingers, turning the glove inside out. Do not remove the inner cotton glove liners at this time. Carefully lower the arms across the chest after the outer gloves have been removed (Figure G-6). Do not allow the patient's arms to come into contact with the exterior of his overgarment. Drop his gloves into the contaminated waste bag. Dip your gloves in the 5 percent hypochlorite solution.
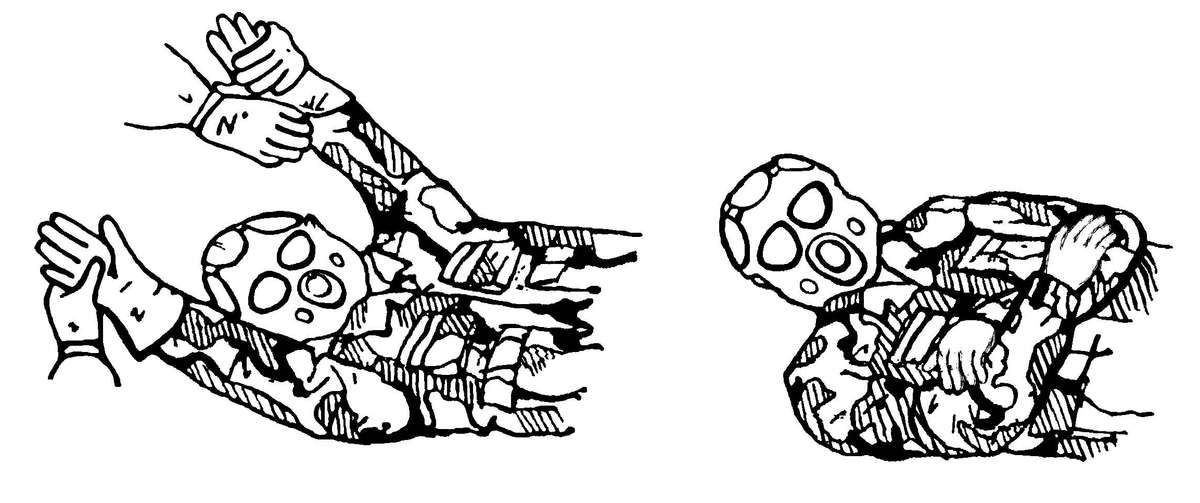
Figure G-6. Remove outer gloves and position arms after glove removal.
(6) Remove overboots. Cut the overboot laces and fold the lacing eyelets flat outwards. If the green vinyl overboot (GVO) is worn, first try to remove the overboot without cutting; if necessary, cut the boot along the front. While standing at the foot of the litter, hold the heel with one hand, pull overboot downwards, and then pull towards you to remove the overboot over the combat boot heel. Remove the two overboots simultaneously. This reduces the likelihood of contaminating one of the combat boots. While holding the heels off the litter, have a decontamination team member wipe the end of the litter with the 5 percent hypochlorite solution to neutralize any liquid contamination that was transferred to the litter from the overboots. Lower the patient's heels onto the decontaminated litter. Place the overboots in the contaminated waste bag. Decontamination personnel dip their gloves in the 5 percent hypochlorite solution.
e. Step 5. Remove patient's battle dress uniform.
(1) Remove battle dress uniform. Cut the BDU jacket and trousers as described above for the protective overgarment. Roll the jacket and trousers as described for the protective overgarment.
(2) Remove combat boots. Cut the bootlaces along the tongue. Remove the boots by pulling them towards you. Place the boots in the contaminated waste bag. Do not touch the patient's skin with contaminated gloves when removing his boots.
(3) Remove undergarments. Remove the patient's tee shirt. Dip the cutting device in the 5 percent hypochlorite solution between each cut. Cut both sleeves from the inside, starting at the elbow, up to the armpit. Continue cutting across the shoulder to the collar. Cut around bandages or splints, leaving them in place. Next, peel the tee shirt away from the body to avoid spreading contamination. If the patient is wearing a brassiere, cut it between the cups. Cut both shoulder straps where they attach to the cups and lay them back off of the shoulders. Remove the patient's under shorts/panties by cutting from the lower side of the hip to the waist on both sides. Fold the front flap of the shorts/panties down between the patient's legs onto the litter. Do not allow the outside of the garment to touch the patient's skin. Remove the socks and cotton glove liners. Do not remove the patient's identification tags.
f. Step 6. Transfer the patient to a decontamination litter. After the patient's clothing has been cut away, he is transferred to a decontamination litter or a canvas litter with a plastic sheeting cover. Three decontamination team members decontaminate their gloves and aprons with the 5 percent hypochlorite solution. One member places his hands under the patient's legs at the thighs and Achilles tendons, a second member places his arms under the patient's back and buttocks, and a third member places his arms under the patient's shoulders and supports the head and neck. They carefully lift the patient using their knees (not their backs) to minimize back strain. While the patient is elevated, another decontamination team member removes the litter from the litter stands and replaces it with a decontaminated (clean) litter. The patient is carefully lowered onto the clean litter. The contaminated clothing and overgarments are placed in bags and moved to the contaminated waste dump. The dirty litter is rinsed with the 5 percent hypochlorite solution and placed in the litter storage area.
g. Step 7. Decontaminate skin.
(1) Spot decontamination. With the patient in a supine position, spot decontaminate the skin using the SDK or a 0.5 percent hypochlorite solution. Decontaminate areas of potential contamination. Include areas around the neck, wrists, and lower parts of the face. Decontaminate the patient's identification tags and chain, if necessary.
NOTE
Complete body wash is not appropriate and may be injurious to the patient. During complete body wash, the patient would have to be rolled over to reach all areas of the skin. This is not necessary for adequate decontamination.
(2) Trauma specialist care. During decontamination, the clothing around bandages, tourniquets, and splints was cut and left in place.
WARNINGS
1. DO NOT apply the SDK or irrigate wounds in the abdominal and thoracic cavities or intracranial head injuries.
2. DO NOT remove splints.
(3) Check patient for completeness of decontamination. The patient is checked with the CAM or with M8 detector paper for completeness of decontamination.
NOTE
Other monitoring devices may be used when available.
(4) Dispose of contaminated waste. Dispose of contaminated bandages and coverings by placing them in a contaminated waste bag. Seal the bag and place it in the contaminated waste dump.
h. Step 8. Transfer the patient across the shuffle pit.
(1) The patient's clothing has been cut away; his skin, bandages, and splints have been decontaminated. Now the litter is transferred to the shuffle pit and placed upon the litter stands. The shuffle pit is wide enough to prevent the patient decontamination team members from straddling it while carrying the litter. Four decontamination team members transfer the patient to a clean treatment litter in the shuffle pit. A member of the patient decontamination team removes the bagged FMC and holds it so that a trauma specialist on the clean side of the hot line can read it. A trauma specialist on the clean side of the hot line prepares a new FMC before the patient is moved to the clean area. The old FMC is disposed of with other contaminated waste.
(2) Decontamination team members rinse or wipe down their aprons and gloves with the 5 percent hypochlorite solution.
(3) Three decontamination team members lift the patient off the decontamination litter (see Step 6 for lifting procedures).
(4) While the patient is elevated, another decontamination team member removes the litter from the stands and returns it to the decontamination area. A trauma specialist from the clean side of the shuffle pit replaces the litter with a clean one. The patient is lowered onto the clean litter. Two trauma specialists from the clean side of the shuffle pit move the patient to the clean treatment area. The patient is treated in this area or waits for processing into the CPS. The litter removed by the decontamination team member is wiped down with the 5 percent hypochlorite solution in preparation for reuse.
NOTE
Before decontaminating another patient, each decontamination team member drinks approximately one-half quart of water. The exact amount of water consumed is increased or decreased according to the temperature (see Table G-2 below).
Table G-2. Heat Injury Prevention and Water Consumption.
| EASY WORK | MODERATE WORK | HARD WORK | |||||
|---|---|---|---|---|---|---|---|
| HEAT CATEGORY | WBGT INDEX DEGREES F | WORK/ REST MIN | WATER INTAKE QT/HR | WORK/ REST MIN | WATER INTAKE QT/HR | WORK/ REST MIN | WATER INTAKE QT/HR |
| 1 (WHITE) | 78-81.9 | NL | ½ | NL | ¾ | 40/20 | ¾ |
| 2 (GREEN) | 82-84.9 | NL | ½ | 50/10 | ¾ | 30/30 | 1 |
| 3 (YELLOW) | 85-87.9 | NL | ¾ | 40/20 | ¾ | 30/30 | 1 |
| 4 (RED) | 88-89.9 | NL | ¾ | 30/30 | ¾ | 20/40 | 1 |
| 5 (BLACK) | >90 | 50/10 | 1 | 20/40 | 1 | 10/50 | 1 |
| THE WORK/REST TIMES AND FLUID REPLACEMENT VOLUMES WILL SUSTAIN PERFORMANCE AND HYDRATION FOR AT LEAST 4 HOURS OF WORK IN THE SPECIFIED HEAT CATEGORY. | |||||||
| NL=NO LIMIT TO WORK TIME PER HOUR. | |||||||
| REST MEANS MINIMAL PHYSICAL ACTIVITY (SITTING OR STANDING) ACCOMPLISHED IN SHADE, IF POSSIBLE. | |||||||
| CAUTION: HOURLY FLUID INTAKE SHOULD NOT EXCEED 1 QUART. | |||||||
| DAILY FLUID INTAKE SHOULD NOT EXCEED 12 QUARTS. | |||||||
| WEARING BODY ARMOR ADDS 5° F TO WBGT INDEX. | |||||||
| WEARING ALL MOPP OVERGARMENTS ADDS 10° F TO WBGT INDEX. | |||||||
WARNING
Do not exceed a fluid intake of 1 quart per hour. Do not exceed a fluid intake of 12 quarts per day.
a. All ambulatory patients requiring EMT or ATM in the clean area of the BAS will be decontaminated. A member of the decontamination team or other ambulatory patients will assist the patient in removing his clothing and decontaminating his skin.
b. Patients requiring only minimal care will undergo spot decontamination of their MOPP gear as required for their medical treatment. They will be treated in the contaminated EMT area and returned to duty. They will undergo decontamination and a MOPP gear exchange with their unit.
c. Stable patients not requiring treatment at the BAS, but requiring evacuation to a higher level of care for treatment (example: A patient with a broken arm) should be evacuated in MOPP Level 4 by any available transportation. However, before evacuation, spot remove all thickened/persistent agents from protective clothing.
NOTES
1. Remember, do not remove clothing from an ambulatory patient unless he requires treatment in the clean treatment area of the BAS or clearing station. Only spot decontaminate the patient's clothing and evacuate him to the next level of care.
2. Place cutting device used in this procedure in a container of 5 percent hypochlorite solution when not in use. Most ambulatory patients will be treated in the contaminated treatment area and returned to duty. Upon removal of an ambulatory patient's clothing, he becomes a litter patient. The BAS and clearing station do not have clothing to replace those cut off during the decontamination process. The patient must be placed in a PPW for protection during evacuation. A battery operated blower unit with a CB filter may be attached to the PPW to provide fresh air to the patient; thus reducing the carbon dioxide buildup inside the PPW (Figure G-7).
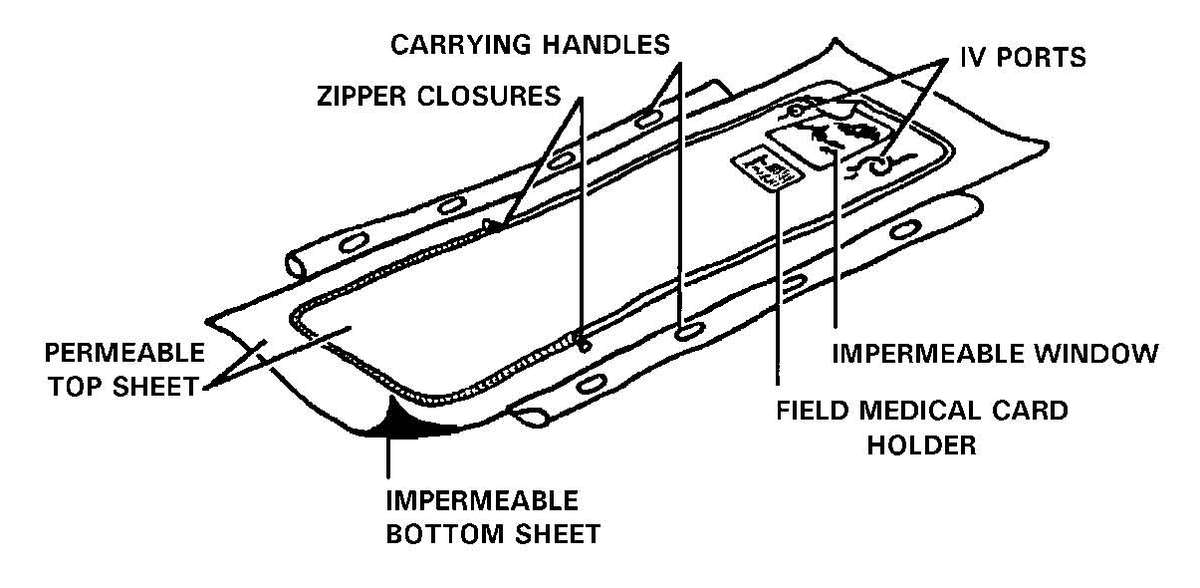
Figure G-7. Chemical warfare agent protective patient wrap.
d. Step 1. Remove load-carrying equipment. Remove load-carrying equipment (LCE) by unfastening/unbuttoning all connectors or tie straps; then place the equipment in a plastic bag. Place the plastic bag in the designated storage area for later decontamination.
e. Step 2. Decontaminate the patient's mask and hood. After the patient has been triaged and treated (if necessary) by the senior trauma specialist in the PDS, the patient (assisted by another ambulatory patient or a member of the patient decontamination team, if necessary) begins the clothing removal process.
(1) Decontaminate and remove mask hood. Sponge down the front, sides, and top of the hood with a 5 percent hypochlorite solution. Remove the hood by cutting (Figure G-3) or, with the quick-doff hood or other hoods, by loosening the hood from the mask attachment points. Before cutting the hood, dip the cutting device in the 5 percent hypochlorite solution. Begin by cutting the neck cord and the small string under the voicemitter. Next, release or cut the hood shoulder straps and unzip the hood zipper. Proceed by cutting the hood upward, close to the filter inlet cover and eye-lens outserts, to the top of the eye-lens outsert, across the forehead to the outer edge of the other eye-lens outsert. Proceed downward toward the patient's shoulder, staying close to the eye-lens and filter inlet. Cut across the lower part of the voicemitter to the zipper. After dipping the cutting device in the 5 percent hypochlorite solution again, cut the hood from the center of the forehead over the top of the head and fold the right and left sides of the hood away from the patient's head, removing the hood.
(2) Decontaminate the mask and patient's face. Decontaminate the mask and the patient's face by using the SDK or a 0.5 percent hypochlorite solution. Wipe the external parts of the mask; cover both mask air inlets with gauze or your hands to keep the mask filters dry. Continue by wiping the exposed areas of the patient's face, to include the neck and behind the ears.
f. Step 3. Remove Field Medical Card. Cut the FMC tie wire, allowing the card to fall into a plastic bag. Seal the plastic bag and rinse it with the 5 percent hypochlorite solution. Place the plastic bag under the back of the protective mask head straps.
g. Step 4. Remove all gross contamination from the patient's overgarment. Remove all visible contamination spots by using the SDK (preferred method) or a sponge dipped in a 5 percent hypochlorite solution.
h. Step 5. Remove overgarments.
(1) Remove the patient's personal effects. Place the patient's personal effects in a clean bag and label with the patient's identification. If they are not contaminated, give them to him. If his personal effects are contaminated, place the bagged items in the contaminated storage area until they can be decontaminated, then return them to the patient.
(2) Remove overgarment jacket. Have the patient stand with his feet spread apart at shoulder width. Unsnap the jacket front flap and unzip the jacket. If the patient can extend his arms, have him clinch his fists and extend his arms backward at about a 30° angle. Move behind the patient, grasping his jacket collar at the sides of the neck, peel the jacket off the shoulders at a 30° angle down and away from the patient. Avoid any rapid or sharp jerks that can spread contamination. Gently pull the inside sleeves [G-16] over the patient's wrists and hands. If the patient cannot extend his arms, you must cut the jacket to aid in its removal. Dip the cutting device in the 5 percent hypochlorite solution between each cut. As with the litter patient, cut both sleeves from the inside, starting at the wrist, up to the armpit. Continue cutting across the shoulder to the collar. Cut around bandages or splints, leaving them in place. Next, peel the jacket back and downward to avoid spreading contamination. Ensure that the outside of the jacket does not touch the patient or his inner clothing.
(3) Remove overgarment trousers. Unfasten or cut all ties, buttons, or zippers before grasping the trousers at the waist and peeling them down over the patient's combat boots. Again, the trousers are cut to aid in removal. If necessary, cut both trouser legs starting at the ankle, keeping the cuts near the inside of the legs, along the inseam, to the crotch. Cut around all bandages, tourniquets, or splints. Continue to cut up both sides of the zipper to the waist and allow the narrow strip with the zipper to drop between the legs. Place the cutting device in the 5 percent hypochlorite solution. Peel or allow the trouser halves to drop to the ground. Have the patient step out of the trouser legs, one at a time. Place the trousers in the contaminated disposal bag.
(4) Remove overboots. Remove the patient's overboots by cutting the laces with cutting device dipped in the 5 percent hypochlorite solution. Fold the lacing eyelets flat on the ground. Step on the toe and heel eyelets to hold the overboot on the ground and have the patient step out of it. Repeat this procedure for the other overboot. If the GVO are worn, first try to remove the overboots without cutting; if necessary, cut the overboots along the front. If the overboots are in good condition, they can be decontaminated and reissued.
(5) Remove the patient's outer gloves. Grasp the heel of the glove, peel the glove off with a smooth downward motion. Place the contaminated gloves in a plastic bag with the overgarment jacket. Do not allow the patient to touch his clothing or other contaminated objects with his exposed hands.
(6) Remove the patient's cotton glove liners. Have the patient remove his cotton glove liners to reduce the possibility of spreading contamination. Have the patient grasp the heel of one glove liner with the other gloved hand, peeling it off of his hand. Hold the removed glove by the inside and grasp the heel of the other glove, peeling it off of his hand. Place both glove inserts in the contaminated waste bag.
i. Step 6. Remove patients BDU.
(1) Remove the patient's personal effects. Place the patient's personal effects in a clean bag and label with the patient's identification. If they are not contaminated, give them to him. If his personal effects are contaminated, place the bagged items in the contaminated storage area until they can be decontaminated, then return them to the patient.
(2) Remove BDU jacket. Have the patient stand with his feet spread apart at shoulder width. Unbutton the front flap of the jacket. If the patient can extend his arms, have him clinch his fists and extend his arms backward at about a 30° angle. Move behind the patient, grasping his jacket collar at the sides of the neck, peel the jacket off the shoulders at a 30° angle down and away from the patient. Avoid any rapid or sharp jerks that can spread contamination. Gently pull the inside sleeves over the patient's wrists and hands. If the patient cannot extend his arms, you must cut the jacket to aid in its removal. Dip [G-17] the cutting device in the 5 percent hypochlorite solution between each cut. As with the litter patient, cut both sleeves from the inside, starting at the wrist, up to the armpit. Continue cutting across the shoulder to the collar. Cut around bandages or splints, leaving them in place. Next, peel the jacket back and downward to avoid spreading contamination. Ensure that the outside of the jacket does not touch the patient or his inner clothing.
(3) Remove BDU trousers. Unfasten or cut all ties, buttons, or zippers before grasping the trousers at the waist and peeling them down over the patient's combat boots. Again, the trousers are cut to aid in removal. If necessary, cut both trouser legs starting at the ankle, keeping the cuts near the inside of the legs, along the inseam, to the crotch. Cut around all bandages, tourniquets, or splints. Continue to cut up both sides of the zipper to the waist and allow the narrow strip with the zipper to drop between the legs. Place the cutting device in the 5 percent hypochlorite solution. Peel or allow the trouser halves to drop to the ground. Have the patient step out of the trouser legs, one at a time. Place the trousers in the contaminated disposal bag.
(4) Remove undergarments. Remove the patient's tee shirt. Dip the cutting device in the 5 percent hypochlorite solution between each cut. Cut both sleeves from the inside, starting at the elbow, up to the armpit. Continue cutting across the shoulder to the collar. Cut around bandages or splints, leaving them in place. Next, peel the tee shirt away from the body to avoid spreading contamination. If the patient is wearing a brassiere, cut it between the cups. Cut both shoulder straps where they attach to the cups and lay them back off of the shoulders. Remove the patient's under shorts/panties by cutting from the lower side of the hip to the waist on both sides. Allow the shorts/panties to fall to the ground. Do not remove the patient's identification tags.
j. Step 7. Check patient for contamination. After the patient's BDU and underwear has been removed check the skin for contamination by using M8 detector paper or the CAM. Carefully survey all areas of the patient's skin, paying particular attention to areas around the neck, wrist, ears, and dressings, splints, or tourniquets.
k. Step 8. Decontaminate skin.
(1) Spot decontamination. Use the SDK or the 0.5 percent hypochlorite solution to spot decontaminate exposed neck and wrist areas, splints, other areas where the protective overgarment was damaged, and where dressings or bandages were removed. Decontaminate the patient's identification tags, if necessary. Have the patient hold his breath and close his eyes. Have him, or assist him, lift his mask at the chin. Wipe his face with the M291 pad or the 0.5 percent hypochlorite solution. Wipe quickly from below the top of one ear, being careful to wipe all folds of the skin, top of the upper lip, chin, dimples, earlobes, and nose. Continue up the other side of the face to the top of the other ear. Wipe the inside of the mask where it touches the face. Have the patient reseal and check his mask.
CAUTION
Keep the decontamination solution out of the patient's eyes.
(2) Trauma specialist care. During clothing removal, the clothing around bandages, tourniquets, and splints was cut and left in place.
l. Step 9. Dispose of contaminated waste. Dispose of contaminated bandages and coverings by placing them in a plastic bag and sealing the bag with tape. Place the plastic bags in the contaminated waste dump.
m. Step 10. Proceed through the shuffle pit to the clean treatment area. Have the decontaminated patient proceed through the shuffle pit to the clean treatment area. Make sure that the patient's boots are thoroughly decontaminated by stirring the contents of the shuffle pit with his boots as he crosses it. The patient will remove his combat boots and socks at the entrance of the clean treatment area or CPS; remove the protective mask at the entrance to the clean treatment area or inside the ambulatory air lock of the CPS.
The decontamination station as established for chemical agent patients is also used for biologically contaminated patients. The eight-man patient decontamination team is required for biologically contaminated patient decontamination procedures.
a. Remove the patient's personal effects. Place the patient's personal effects in a clean bag and label with the patient's identification. If they are not contaminated, give them to him. If his personal effects are contaminated, place the bagged items in the contaminated storage area until they can be decontaminated, and then return them to the patient.
b. Remove the Field Medical Card. Remove the FMC by cutting the tie wire and allowing the FMC to drop into a plastic bag. Keep the FMC with the patient.
c. Remove the patient's clothing. Patient decontamination team members first apply the 5 percent hypochlorite solution to the patient's clothing and the litter. Then, remove the patient's clothing as in decontamination of chemical agent patients. Bandages, tourniquets, and splints are not removed. Move patient to a clean litter as described for a chemical agent patient. Place patient's clothing in a plastic bag and dispose in the contaminated waste dump.
d. Decontaminate the patient's skin. Bathe the patient with soap and warm water or apply the 0.5 percent hypochlorite solution. The trauma specialist places a new tourniquet ½ to 1 inch above the old tourniquet, and then he removes the old one. The trauma specialist removes bandages and decontaminates the skin and wound with the 0.5 percent hypochlorite solution; he replaces the bandage, if needed, to control hemorrhage. Splints are disinfected by soaking the splint, cravats, and straps with the 0.5 percent hypochlorite solution.
NOTE
Use a 0.5 percent hypochlorite solution to decontaminate patients suspected of being contaminated with mycotoxins.
e. Transfer patient to hot line. Two decontamination team members move patient to the hot line. Request assistance from two other decontamination team members to transfer him to a clean litter as described for chemical agent patients. Place the patient's FMC in the plastic bag on the clean litter with him. Two trauma specialists from the clean side of the hot line move the patient from the hot line to the clean treatment/holding area.
a. Remove the patient's personal effects. Place the patient's personal effects in a clean bag and label with the patient's identification. If they are not contaminated, give them to him. If his personal effects are contaminated, place the bagged items in the contaminated storage area until they can be decontaminated, then return them to the patient.
b. Remove the Field Medical Card. Remove the FMC by cutting the tie wire and allowing the FMC to drop into a plastic bag. Keep the FMC with the patient.
c. Remove the patient's clothing. Patient decontamination team members first apply the 5 percent hypochlorite solution to the patient's clothing. Then remove the patient's clothing as in decontamination of chemical agent patients. Bandages, tourniquets, and splints are not removed. Place patient's clothing in a plastic bag and dispose in the contaminated waste dump.
d. Decontaminate the patient's skin. Have the patient bathe with soap and warm water or apply the 0.5 percent hypochlorite solution. If the patient is unable to bathe himself, a member of the [G-20] decontamination team must bathe him. The trauma specialist places a new tourniquet ½ to 1 inch above the old tourniquet, and then he removes the old one. The trauma specialist removes bandages and decontaminates the skin and wound with the 0.5 percent hypochlorite solution; he replaces the bandage, if needed, to control hemorrhage. Splints are disinfected by soaking the splint, cravats, and straps with the 0.5 percent hypochlorite solution.
NOTE
Use a 0.5 percent hypochlorite solution to decontaminate ambulatory patients suspected of being contaminated with mycotoxins.
e. Direct patient across hot line. Direct the patient to cross the hot line to the clean treatment area. His boots must be decontaminated at the hot line before he enters the clean treatment area.
NOTES
1. Remember, do not remove clothing from an ambulatory patient unless he requires treatment in the clean treatment area of the BAS or clearing station. Only spot decontaminate the patient's clothing and evacuate him to the next level of care.
2. Place cutting device used in this procedure in a container of 5 percent hypochlorite solution when not in use. Most ambulatory patients will be treated in the contaminated treatment area and returned to duty. Upon removal of an ambulatory patient's clothing, he becomes a litter patient. The BAS and clearing station do not have clothing to replace those cut off during the decontamination process. The patient must be placed in a PPW for protection during evacuation (Figure G-7).
The practical decontamination of nuclear-contaminated patients is easily accomplished without interfering with the required medical care.
NOTE
Patients must be monitored by using a radiac meter (AN/VDR2, AN/PDR27, or AN/PDR77) before, during, and after each step of the decontamination procedure.
a. Remove patient's personal effects. Patient decontamination team members remove the patient's personal effects and place them in a plastic bag. Place plastic bag in a clean holding area.
b. Remove patient's clothing. Patient decontamination team members remove the patient's outer clothing as described for chemical agent patients. Do not remove bandages, tourniquets, or splints. Move the patient to a clean litter. Place the patient's contaminated clothing in a plastic bag and move the bagged clothing to the contaminated waste dump.
NOTE
Patients arriving at the MTF in MOPP will only have their MOPP removed. They can remain in their BDU unless contamination is found on it.
c. Spot decontaminate patient's skin. Wash exposed skin surfaces with soap and warm water. Wash the hair with soap and warm water, or clip the hair and wash the scalp with soap and warm water.
d. Transfer patient to hot line. Move the patient to the hot line. Two trauma specialists from the clean side of the hot line move the patient into the clean treatment area.
a. Remove patient's personal effects. Have the patient remove his personal effects and place them in a plastic bag.
b. Remove patient's outer clothing. Have the patient remove his outer clothing (or have a decontamination team member assist him). Place his contaminated clothing in a plastic bag and move the bagged clothing to the contaminated waste dump.
NOTE
Patients arriving at the MTF in MOPP will only have their MOPP removed. They can remain ambulatory in their BDU unless contamination is found on it.
c. Spot decontaminate patient's skin. Have the patient wash his exposed skin surfaces with soap and warm water. Wash his hair with soap and water, or clip the hair and wash the scalp with soap and water.
d. Transfer patient to hot line. Direct the patient to move to the hot line. Decontaminate his boots by stirring the shuffle pit contents with his feet before he crosses into the clean treatment area.
NOTE
If a new protective overgarment is not available, after treatment, the ambulatory patient must be placed in a PPW for protection during MEDEVAC to the next level of care MTF. Thus, he becomes a litter patient for evacuation.
Medical units must have protection from NBC attack and contamination to survive and function effectively. The extent of protection provided is only limited by the resources available and efforts of unit personnel. Protection as simple as an individually dug foxhole or as elaborate as the subbasement of a concrete building may be used. Expedient protection from the effects of biological and chemical agents are usually much less labor intensive.
The level of protection from radiation is expressed in terms of shielding. Material is available on the battlefield to construct/prepare expedient fallout shelters that offer substantial shielding against gamma radiation (see Table H-1). Generally, the denser or heavier the material, the better shielding it offers. The degree of protection afforded by a fallout shelter is expressed as a "protection factor," or a "transmission factor." The protection factor is simply the fraction of the available radiation dose that penetrates the shelter and reaches those inside compared to the radiation received by an unprotected person. Thus, a protection factor of 2 indicates that an individual in the shelter receives one-half of the radiation dose he would receive if unprotected. A protection factor of 100 (associated with about six half-value thicknesses) indicates that only 1/100 or 1 percent of the radiation dose reaches the inside. Transmission factors are expressed in percentages, or in decimals. Either refers to that fraction of the ambient unshielded dose that is received by personnel within the shelter. Fallout gamma transmission factors for some common shelters are shown in Table H-2.
Table H-1. Shielding Potential of Common Materials—Fallout Gamma Protection
| MATERIAL | 1/2 VALUE LAYER THICKNESS[*] | |||
|---|---|---|---|---|
| STEEL | 1.8 CM | (.7") | ||
| CONCRETE | 5.6 CM | (2.2") | ||
| EARTH | 8.4 CM | (3.3") | ||
| WATER | 12.2 CM | (4.8") | ||
| WOOD | 22.4 CM | (8.8") | ||
[*] 1/2 VALUE LAYER THICKNESS—THICKNESS OF A GIVEN MATERIAL WHICH REDUCES THE DOSE OR DOSE RATE TO APPROXIMATELY ONE-HALF OF THAT FALLING UPON IT. | ||||
Table H-2. Transmission factors for Nuclear Radiation[*]
| INITIAL | |||
|---|---|---|---|
| ENVIRONMENTAL SHIELDING | NEUTRONS | GAMMA | RESIDUAL |
| BUILT-UP CITY AREA (IN OPEN) | 1.0 | 0.5 | 0.7 |
| FOXHOLES | 0.3 | 0.2 | 0.1 |
| FRAME HOUSE: | |||
| FIRST FLOOR | 1.0 | 0.9 | 0.5 |
| BASEMENT | 0.5 | 0.3 | 0.1 |
| MULTISTORY BUILDINGS: | |||
| TOP FLOOR | 1.0 | 0.9 | 0.1 |
| INTERMEDIATE FLOORS | 0.9 | 0.9 | 0.02 |
| LOWER FLOOR | 0.9 | 0.5 | 0.1 |
| BASEMENT | 0.5 | 0.3 | 0.01 |
| SHELTER, CLOSED 91 CM (3 FT) | |||
| (EARTH COVER) | 0.05 | 0.02 | 0.005 |
| ARMORED VEHICLES: | |||
| ARMORED PERSONNEL CARRIER | 0.3 | 0.2 | 0.1 |
| TANKS | 0.3 | 0.2 | 0.1 |
| WOODED FOREST | 1.0 | 1.0 | 0.8 |
| [*] INSIDE DOSE = TRANSMISSION FACTOR TIMES OUTSIDE DOSE. | |||
a. In many cases it will be unnecessary to construct field expedient or other types of fallout shelters. There are many structures and terrain features available that afford a degree of fallout protection. Existing fallout shelters are tunnels, caves, culverts, overpasses, ditches, ravines, and man-made structures. The best existing shelters are basements. Figure H-1 shows typical protection provided in buildings. Windows can be sandbagged or covered with dirt from the outside to provide additional protection.
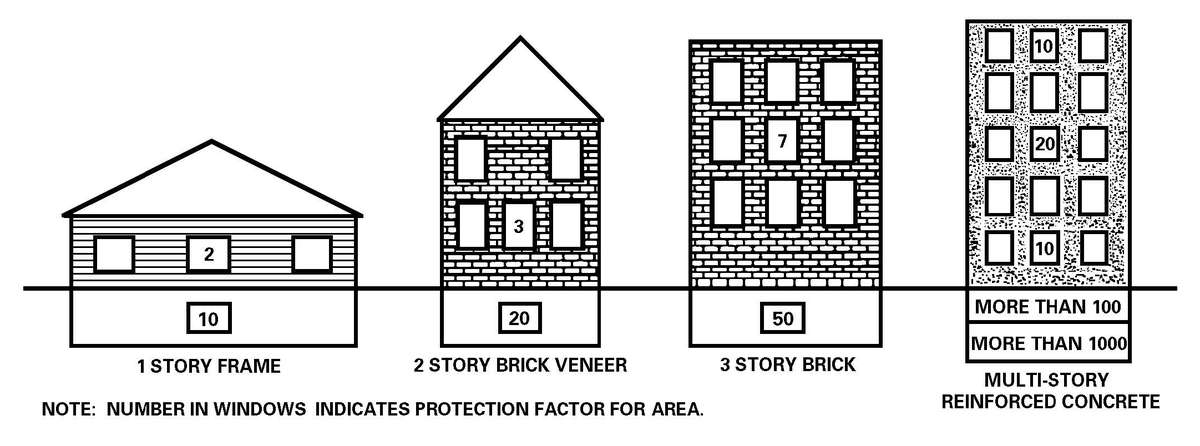
Figure H-1. Typical shelter protection provided in buildings.
b. Planners should attempt to locate HSS units near existing shelters, whenever possible. However, if an HSS unit is already established, or must be established where fallout shelters are not available, then a shelter must be constructed. Elaborate shelters are not required, since they usually only need to be occupied for a few days. There are a number of field expedients that will serve to save personnel and patients even though they may not be comfortable for those few days.
c. When engineer support is available, a bulldozer trench about 2.7 meters (9 feet) wide and 1.2 meters (4 feet) deep can be dug (Figure H-2). The length of the trench will be determined by the number of patients/personnel to be sheltered. About 0.6 meter (2 feet) length of trench is required for each person to be sheltered. These trenches reduce exposure of personnel lying on the floor to about 20 to 30 percent of the radiation that they would receive in the open. Protection and comfort can be improved, as time permits, by digging the trenches deeper; undercutting the walls (care must be taken in this option; the earth may cave in); erecting tents over the trenches; and providing improved flooring. When used with other individual and collective protection measures, bulldozer trenches provide adequate fallout shelters for most situations; they can be provided in a minimum of time and effort. Trenches should not be dug in areas subject to flooding during rainstorms; a berm should be formed on the uphill side of the trench to direct water around the trench in the event rainfall occurs in the area. Undercutting will not be possible in sandy soil; also some form of support to keep the walls from caving in is required.
d. Dug-in tents (Figure H-3) for hospitals provide more comfort and require less movement than the bulldozer trench; however, they have two drawbacks. First, they offer far less radiation protection than the bulldozer trench, and second, they require considerably more engineer effort. This option should work well with GP tents, but will probably be hard to accomplish with the TEMPER.
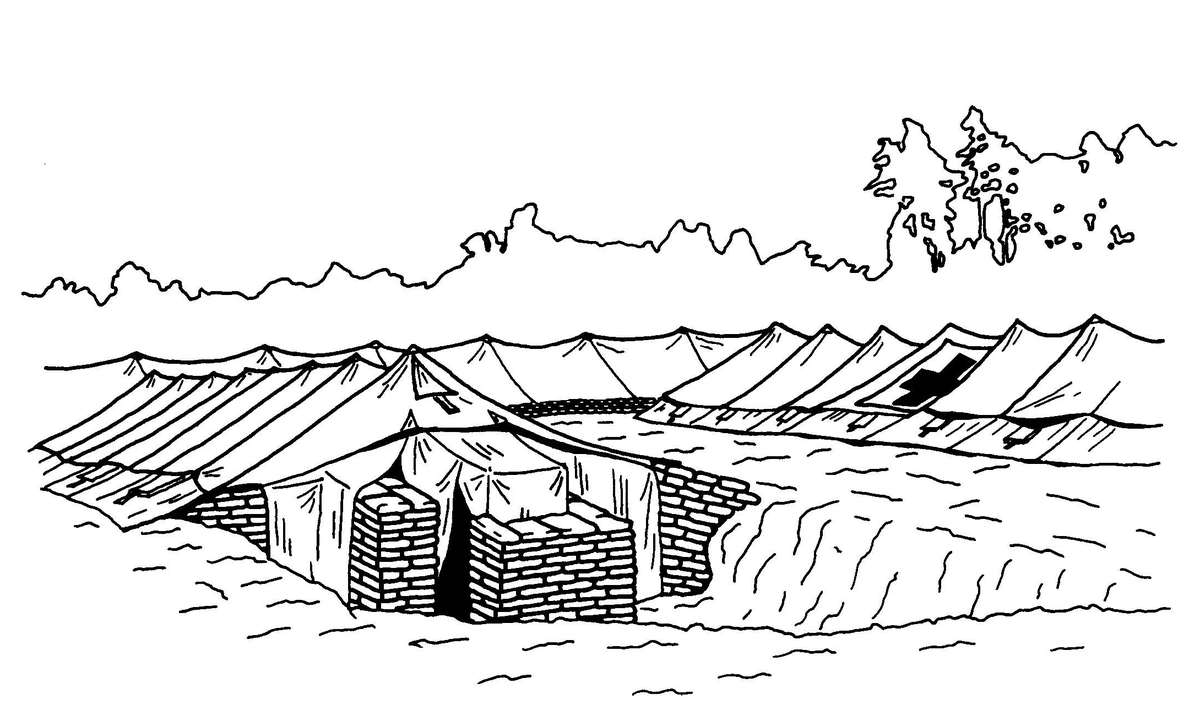
e. Sandbagged walls around the hospital tents, as shown in Figure H-4, or lightly constructed buildings provide protection from fallout. Sandbagged walls 1.2 meters high give significant protection (20 to 40 percent transmission factor); however, the effort required to achieve the protection is such that it is marginally feasible. Sandbagging is an effective means for supplementing other shelters by—
f. When other shelters are not available, HSS units must prepare foxholes and trenches for patients and unit personnel. As time permits, improve these shelters by deepening, covering, undercutting, and sandbagging.
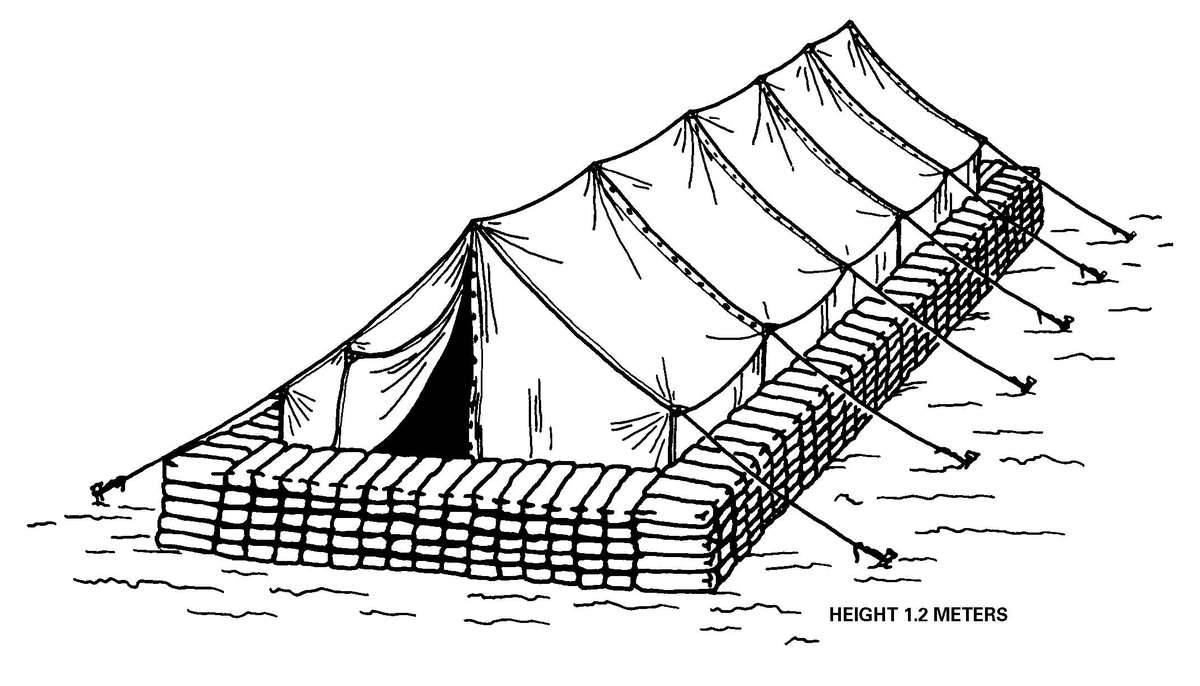
Figure H-4. Sandbag walls around tents.
a. When CPS systems are not available, well-sealed shelters (TEMPER, ISO, and GP) can significantly minimize or prevent the entry of CB agents. The ventilation system must be turned off, and kept off, before, during, and after the attack. The shelter must be totally sealed during this time to maximize protection. Table H-3 provides examples of protection values for well-sealed shelters. For example, a well-sealed TEMPER will only permit 1/60 of the CB agent outside to enter the shelter. If a persistent agent is used, be aware of agent off-gassing hazards. Persistent agents can penetrate TEMPER fabric and create a vapor hazard inside. In a CB agent attack, ensure that all staff and patients are protected by wearing their MOPP or are in PPWs.
Table H-3. Ratio at Nonpersistent Agent Concentrations (Inside/Outside) for Different Shelters
| SHELTER | RATIO INSIDE/ OUTSIDE |
|---|---|
| TEMPER TENT | 1:60[*] |
| GENERAL PURPOSE TENT, MEDIUM, WITH COTTON LINER | 1:50 |
| GENERAL PURPOSE TENT, LARGE, WITH COTTON LINER | 1:30 |
| ISO SHELTER | 1:60 |
| [*] THE VENTILATION SYSTEM MUST BE TURNED OFF ON ALL SHELTERS TO PROVIDE THIS LEVEL OF PROTECTION. | |
b. Sealing shelters to prevent entry of CB agents does not require elaborate materials or procedures.
(1) Materials needed for sealing shelters include, but are not limited to the following:
(2) All vulnerable areas must be sealed. Seal—
NOTES
1. Do not allow any entries/exits to shelters during a CB attack.
2. In hot climates the heat load will rise in sealed shelters with the ventilation system turned off. Personnel must carefully monitor each other and the patients. All personnel must drink plenty of water to prevent heat injuries; see FM 21-10.
Water supplies in areas with NBC contamination and in surface water supplied by runoff from such areas will most likely be contaminated. The contamination of water, whether intentional or inadvertent, may reach concentrations that will produce casualties. By special methods of analysis, the presence of contamination can be determined. Treatment of contaminated water requires chemicals and equipment that are only available to quartermaster water purification units; individuals or units should not attempt to treat their water. Decontamination of water is only undertaken when uncontaminated sources are not available; then ONLY with the approval of the medical authority (PVNTMED or surgeon).
a. Detection of nuclear contamination in water is accomplished by using the AN/PDR77, AN PDR/27 or AN VDR/2 radiac meters.
CAUTION
DO NOT allow the probe to come into contact with the water source; allow at least one inch of air space between the probe and water surface.
b. Detection of BW agents in water is accomplished by the use of field biological water test kits and specially designed collection and detection kits. The specialty kits will be provided as needed, and will be available to PVNTMED and supporting medical laboratory personnel. When required for the President and Secretary of Defense purpose, samples must be collected and prepared for shipment to the supporting medical laboratory. A chain of custody document must be prepared by the collector and maintained as the sample(s) is being transported to the supporting medical laboratory and throughout its transit to the CONUS laboratory. See Appendix F for details on suspect BW sample collection, packaging, chain of custody, and handling.
c. The Chemical Agent Water Testing Kit, M272, provides a rapid field test to detect chemical agent contamination in water. The test must be conducted before the water is treated with chlorine; the chlorine will affect the accuracy of the test for chemical agents.
When contamination is discovered the following actions are taken:
a. Mark the water source, using the standard NBC contamination markers, and ensure that personnel do not consume the water until approved.
b. Notify the commander that the water source is contaminated and unfit for drinking, food preparation, and personal hygiene.
c. Notify the supporting water production unit, such as the quartermaster water production and distribution unit of the contaminated water source.
d. The commander establishes safeguards to prevent personnel from using the contaminated water supply.
e. An alternative source of uncontaminated water is sought and used. The primary source for obtaining water is from quartermaster-operated water production and distribution points. Other sources are considered only when quartermaster-operated faculties are not available. Alternative sources that may be considered include—
f. Contaminated water must not be used until it has been treated by quartermaster water production and distribution units or other equally capable water purification units and approved for use by the medical authority.
Contaminated water requires additional equipment and supplies to remove the contamination. Quartermaster water purification and distribution units are equipped to perform these duties. See FM 10-52 for details.
a. Food Susceptibility. Stored, transported, and prepared food is susceptible to NBC contamination throughout the TO. Planning for any battle or operation must include food protection from contamination; food contamination detection; and contaminated food disposition (decontaminate or destroy).
b. Countermeasures. There are three primary countermeasures to overcome or reduce the NBC hazard to food:
(1) Contamination avoidance.
(2) Nuclear, biological, and chemical agent detection.
(3) Nuclear, biological, and chemical agent decontamination.
c. Priorities. The priorities for conducting NBC countermeasures are—
(1) Contamination avoidance. Contamination avoidance includes using natural and fabricated barriers to prevent, or significantly reduce the spread of contamination. Also, using specific procedures for entry and exit between contaminated and uncontaminated areas reduce the potential for spreading contamination. Use of these barriers and procedures may reduce the subsequent need for detection and decontamination.
(2) Detection, measurement, and identification. These activities are essential for determining the presence, extent, and nature of NBC contamination. This information is essential in identifying the existence of uncontaminated supplies, or decontamination requirements.
(3) Decontamination. Decontamination removes the contaminant and provides food that is safe for consumption.
d. Decontamination. Decontamination efforts require an extensive amount of labor, time, and supplies. The use of hasty decontamination is emphasized. That is, decontaminate just enough to sustain operations and keep fighting, rather than to make a contamination-free environment. Normally, decontamination efforts will be limited to the packaging and packing materials. Food decontamination will only occur in critical situations where other food supplies are not available. Most decontamination is performed in or very near the AO. Before beginning decontamination procedures, divide exposed food items into groups based on protection of item at time of exposure. These groups establish priorities based on ease of decontamination and the ability to monitor the food.
(1) Group I—Canned or packaged items exposed only to a chemical agent vapor.
(2) Group II—Canned or packaged items that are contaminated on the outside with a liquid chemical agent, a biological agent, or radioactive fallout.
(3) Group III—Unpacked or poorly packaged items that have been exposed to any NBC agent.
(4) Group IV—Food contaminated through the food chain.
An adequate defensive posture for a chemical attack will also protect food against biological contamination and radiation fallout.
a. Operational Rations. Operational rations include, but are not limited to, MREs; unit group ration (UGR), A; unit group ration, heat and serve; and medical diet supplement.
(1) Packaging materials and storage methods normally protect these rations. The packaging and packing of operational rations protect the contents from deterioration. As a result, the contents are protected from moisture, to include chemical liquids, chemical vapors, and biological agents. Operational rations delivered to an AO will usually have increased levels of packaging and/or packing protection. Operational rations are substantially protected while contained in the shipping cases, especially if protected with an overlay of fiberboard, shrink wrap, or film wrap.
(2) Enclosed storage is used whenever possible. Refrigerated warehouses, cold storage rooms, and even prefabricated refrigerators and trailers provide excellent protection. Underground shelters, caves, and tunnels that can be made airtight provide maximum NBC protection. Buildings provide protection depending on how well they can be closed and sealed. The basement of a building is a good storage place. However, keep in mind that chemical vapors tend to seek out low-lying areas. Storing rations indoors will protect them from liquid droplet and fallout contamination unless the building is damaged by an attack. Complete protection against chemical vapors is only offered by airtight closed spaces like cold storage facilities.
(3) Chemical protective measures are to be integrated into daily logistical operation to avoid the contamination of operational rations. Maximum use is made of alarm and detection equipment, overhead shelter, shielding materials, and protective covers. Back up stocks of operational rations should be dispersed to minimize the risk of destruction or contamination.
(4) An NBC Protective Cover or similar equipment will help greatly. The NBC Protective Cover is discarded and replaced upon becoming contaminated; it reduces overall decontamination requirements; and it improves the survivability of supplies and equipment. The NBC Protective Cover provides 24-hour protection against liquid chemical contamination. Detection paper used on the NBC Protective Cover will rapidly identify a contaminated cover.
b. Bulk and Fresh Foods.
(1) Field expedient or improvised storage may be the only choice available under high-risk conditions. Expedient storage for food supplies may be a natural or man-made depression lined to protect [J-3] contents against moisture, and then covered with earth and sod. The earth gives good protection against all forms of chemical or biological contamination and nuclear fallout.
(2) Foods are only stored outdoors or in partially protected areas when absolutely necessary. Only cases of foods packed in cans, bottles, or airtight foil or film wraps, and foods packed in sealed boxes or multilayered wrappings can be subjected to exposed storage. Partial protection is provided by open sheds, temporary roofing, or tents. When subsistence must be stored in the open, give as much protection as possible. Protection material may include NBC Protective Covers, tarpaulins, tarpaulin sheds, or any other available covering such as plastic sheeting. Tarpaulins and other treated or waterproof coverings do not prevent contamination by chemical vapors, but they do reduce contamination from liquid agents. Canvas will keep out more than 95 percent of liquid contamination for a short period of time after the attack. The canvas must be removed soon after the attack to prevent the agent from seeping through onto the subsistence; placement of spacers between the covering and the food will greatly reduce this problem. Even the thinnest material will offer some protection and is better than nothing at all. Therefore, food supplies must be covered by whatever material is available.
a. Contamination.
(1) Following a nuclear detonation, food can become contaminated in three ways:
(2) Consumption of food contaminated with radioactive fallout may cause a risk of radiation injuries from internal radiation; that is, radiation from radioactive sources within the body. Most isotopes will pass through the digestive tract or be excreted very quickly. However, the intestinal tract may receive a considerable dose. Some isotopes are more hazardous because they are absorbed from the digestive tract and enter the metabolism of man and animals.
(3) Operational rations are safe when surface decontamination is performed before breaking the package. Operational rations stored close to ground zero may become radioactive from induced radiation. It is more likely, however, that the food will be damaged or destroyed by the blast and thermal effects of the nuclear explosion.
(4) Bulk and fresh food stored in the open without protection will be contaminated. Decontamination is very difficult and time-consuming. Efforts should be made to ensure proper packing to prevent food contamination from radioactive fallout. Packing made from hard and nonporous materials, such as plastic or multilayer cardboard with a smooth surface, should be used. In addition, storage facilities should be enclosed to avoid the entry of fallout. Any material used as a protective cover will give some protection against nuclear fallout. Protection against induced radiation, blast, and thermal effects requires a hardened shelter or underground storage.
(5) Food supplies require protection throughout the chain of production or procurement. Protection of the civilian-based food supply includes countermeasures along the production chain. Meats [J-5] and milk are the most vulnerable products because of the possibility for concentration of radioactive isotopes (Strontium, Cesium, and Iodine). The primary, and possibly the only, protection of animal products is to keep the animals indoors and to avoid contaminated fodder. Immediate slaughter of food animals is recommended if there is a shortage of uncontaminated fodder. Also, food animals exposed to fallout should be considered fit for consumption and slaughtered using routine procedures. Unharvested crops cannot be protected.
b. Inspection and Monitoring.
(1) Fallout close to ground zero, especially after a surface burst, may be visible as dust. The presence of dust is an immediate indicator of contamination. Fallout on unprotected food produces a grittiness that is unpleasant and warns against eating the food. The degree and means of food protection (packaging and storage faculties) must be considered. Food in a building that remains intact should not receive enough contamination to be dangerous when eaten.
(2) Veterinary units have the AN/VDR2 Radiac Set and UDR13 dosimeter to conduct ground or aerial surveys for gamma radioactive contamination levels in an area. The measurement of the external gamma radiation in the fallout area is an indication, but not a quantitative measure, for the degree of hazard from food contamination. These units also use the AN/VDR2 Radiac Set for point detection of gamma and beta radiation sources. Food monitoring is conducted in an area with low background radiation. If the storage area is contaminated, the food must be moved to a cleaner area for monitoring. With the AN/VDR2, the initial food monitoring is performed with the probe cover in place and the probe passed approximately 6 inches from the surface. If the reading is twice the background dose rate, the food is considered contaminated. If the reading is not above the background level but contamination is still suspected, place the probe closer to the food with the beta probe cover off. Monitor meat and fish with the probe cover off; pass the probe approximately one-half inch from the surface of the food.
(3) Monitoring food contaminated through the food chain is more complicated; depending on the detection instrument used, special procedures must be followed. Gamma and beta emitting radionuclides in small volumes may be detected using radiac sets such as the AN/VDR2; however, alpha emitting ones cannot. They are rough instruments and may be used only for screening surface contaminated food. To evaluate the hazards; the isotopes contributing to the radioactivity must be identified. Surface contaminated food will contain a mixture of isotopes with some more hazardous than others, depending upon whether they are used by the body. Milk will contain mostly I-131, Cs-137, Sr-89, and Sr-90. Meat and fish will contain mostly Cs-137. To verify I-131, Cs-137, Sr-89, and Sr-90 contamination, samples must be sent to laboratories equipped to analyze the samples.
(4) All newly selected food supplies must be surveyed. Begin continuous monitoring immediately following receipt of a fallout warning, or when increased levels of radiation are detected by periodic monitoring.
(5) Periodic monitoring is needed to establish baseline levels of background radiation in the environment and various food products. This monitoring is performed during peacetime, when possible, and throughout the time US forces are deployed in a TO.
NOTE
The AN/VDR2 is being replaced by the AN/PDR77 Radiac Set.
c. Decontamination. There are two methods for nuclear decontamination: aging and removing. Aging is the process of allowing natural radiation decay to occur. The time necessary for this decay to take place depends upon the isotopes present; each has a different decay rate (half-life). Aging may not be possible when there is a short food supply. In some instances, such as with induced radioactivity, it may be the only way to decontaminate. Removing nuclear contamination from areas, personnel, food, or moving equipment to another location eliminates the immediate hazard. To determine which decontamination method is required, food supplies are divided into groups. See Table J-1 for additional information on food items and decontamination.
(1) Group II—Food in sealed and dust-proof packing such as cans, jars, fiberboard, and cellophane. These products are easily decontaminated by removing the radioactive dust covering the packing; brush, wash with soap and water, or remove the packing (depending on the type of packing material). If radiation is still detected after removing the dust, repeat the brush/wash procedure and remonitor. If radiation is still present, the food itself is then considered radioactive (induced radiation) and is unfit for consumption. Decontamination of induced radiation is possible only through aging. After aging one to two weeks, the food should be safe for consumption. After surface decontamination, the contents are safe to eat unless the food has induced radiation.
(2) Group III—Unprotected food. The method chosen to decontaminate unprotected food items will depend upon whether or not the food supply is critical. If the food supply is not critical, the contaminated items are isolated and allowed to decontaminate by aging. If the food supply is critical, food with surface contamination can, in principle, be decontaminated by removing the contaminated surface, or by washing.
(3) Some products can be decontaminated by washing, peeling, or trimming the outer skin or leaves. Decontaminate potatoes and hard-skinned fruits and vegetables by washing or scrubbing under running water, followed by peeling or scraping, then washing again. Potatoes, carrots, beets, and turnips can be washed at the supply depot. However, do not wash beans, rice, and onions until they are delivered to the field kitchen; washing reduces their storage quality and shelf life. Citrus fruits, pineapples, corn, peas, beans, melons, pumpkins, cabbage, and nuts can be peeled. Decontaminate cucumbers, tomatoes, cherries, cranberries, grapes, pears, plums, and thin-skinned squash by soaking in a water or detergent solution and rinsing with vigorous agitation or brushing. Apricots, peaches, most berries, asparagus, broccoli, and leafy vegetables cannot be satisfactorily decontaminated because of fuzzy surfaces, irregular shapes, or small size, which makes washing difficult.
Table J-1. Decontamination of Food Supplies
| SURFACE OR MATERIAL | TYPE OF CONTAMINATION | ||
|---|---|---|---|
| CHEMICAL | BIOLOGICAL | NUCLEAR | |
| CANNED, BOTTLED, OR PROTECTED BY IMPERMEABLE CONTAINER. | IMMERSE IN BOILING, SOAPY WATER FOR 30 MINUTES AND RINSE. | WASH WITH SOAP AND WATER, THEN IMMERSE IN DISINFECTANT SOLUTION. (IMMERSE IN BOILING WATER FOR 30 MINUTES. FOOD DISINFECTANT, OR 1/3 CANTEEN CUP OF HOUSEHOLD BLEACH IN 10 GAL OF WATER). | WASH WITH SOAP AND WATER, RINSE. BRUSH, WIPE CHLORINE, FOOD CONTAMINATION FROM SURFACE OF CONTAINER. |
| SPRAY WITH DS2 AND RINSE | |||
| WASH IN HOT, SOAPY WATER, RINSE, AND AERATE. | BOIL IN WATER 15 MINUTES; NOT EFFECTIVE ON TOXINS AND SOME SPORES. | ||
| IMMERSE IN 5% SODIUM CARBONATE (4 LB WASHING SODA IN 10 GAL WATER), RINSE WITH POTABLE WATER. | |||
| IMMERSE IN HOUSEHOLD BLEACH SOLUTION (1/2 GAL BLEACH IN 25 GAL WATER) FOR 30 MINUTES THEN RINSE AND AERATE FOR 10 MINUTES. | |||
| IMMERSE IN HTH SOLUTION (1/2 LB IN 25 GAL WATER) 20 MINUTES, THEN RINSE. | |||
| IMMERSE IN STB SOLUTION (1 LB IN 25 GAL WATER) 30 MINUTES, THEN RINSE. | |||
| IMMERSE IN 2% PERACETIC ACID FOR 10 MINUTES, RINSE, AND AERATE FOR 10 MINUTES. | |||
| NOT CANNED OR IMPERMEABLE CONTAINER. | FOOD KNOWN OR SUSPECTED TO BE CONTAMINATED SHOULD NOT BE CONSUMED UNTIL APPROVED BY VETERINARY PERSONNEL. | BOIL IN WATER 15 MINUTES. COOK. IMMERSE IN OR SPRAY WITH 2% HOUSEHOLD BLEACH SOLUTION. PACKAGED, PEELED, OR PARED FOOD MAY BE IMMERSED OR SPRAYED. |
WASH OR TRIM CONTAMINATION FROM UNPACKAGED FOOD. |
(4) Group IV—Food contaminated through the food chain. It is not practical to decontaminate this food. Meat and milk are the two most common foodstuffs contaminated in this way.
(5) Food animals. Food animals that have been exposed to fallout should be considered fit for consumption and slaughtered using routine inspection and slaughter procedures. In those cases where the animal has been exposed to fallout, but is not scheduled for immediate slaughter, the radiation burden can be reduced by moving the animal to an uncontaminated area (barn if available) and washing it with soap and water. Mild radiation sickness does not necessarily mean that the animals cannot be used for food. If the animals have been exposed to an internal radiation hazard, the meat can be eaten if the internal organs are discarded. Chickens that have eaten radioactive material may lay contaminated eggs, but most of the radioactivity will be concentrated in the shells. The white and yolk will be free of harmful amounts of radiation and can be eaten. Chickens will not lay eggs if the radioactive body burden is large enough that their eggs are unfit to eat.
Table J-2. Traditional Salt Preserving Brine
| MEAT, WHOLE 4-5 KG |
| 25% NaCl (SALT) BRINE 5-LITER BRINE PER KG. |
| KEEP MEAT IN BRINE FOR 3 WEEKS, TEMPERATURE BELOW 10°C. |
| SOAK IN WATER FOR 1-2 DAYS. |
| 65-70% OF CS ACTIVITY WILL BE REMOVED. |
| MEAT, CUT 1-2 KG |
| 25% NaCl BRINE 5-LITER BRINE PER KG. |
| KEEP MEAT IN BRINE FOR 4 DAYS. |
| SOAK IN WATER FOR 4 HOURS. |
| 65-70% OF CS ACTIVITY WILL BE REMOVED. |
| MUTTON/LAMB RIB |
| PIECE OF RIB 1-5 KG. |
| 25% NaCl BRINE 5-LITER BRINE PER KG. |
| KEEP IN BRINE FOR 2 DAYS. |
| SOAK IN WATER FOR 2 HOURS. |
| AIR-DRYING FOR 10 DAYS. |
| SOAK IN WATER FOR 2 HOURS. |
| BOIL IN WATER FOR 3 HOURS. |
| 85-90% CS ACTIVITY WILL BE REMOVED. |
| DECONTAMINATION OF COARSELY CHOPPED MEAT |
| 0.9% NaCl SOLUTION. 2-LITER SOLUTION PER KG. |
| SOAK IN NaCl SOLUTION FOR 10 MIN. |
| 60-70% CS ACTIVITY WILL BE REMOVED. |
| REPEATED PROCEDURES WILL REMOVE THE SAME PERCENTAGE OF CS ACTIVITY. |
| SIX TIMES REPEATED TREATMENT WILL REMOVE NEARLY 100% OF CS ACTIVITY. |
d. Considerations When Decontamination is Not Possible. When food cannot be decontaminated, sealing the product in a wrapping material or container may be needed. Sealing the product can reduce or shield the emanation of the contamination and/or fix the contamination in place. The hazard from [J-10] contaminated food is small compared with that from external gamma radiation. Hungry people or animals should not be denied food because of possible fallout contamination. It is not practicable or desirable to preset maximum permissible limits of gross fallout radioactivity as a basis for judging whether or not food should be used. Common sense must be applied in establishing priorities for distribution of available food. For example, use the least contaminated and the most protected food first; hold milk products for 1 to 2 weeks before use.
a. Contamination. Biological warfare agents exist in the form of toxins and microorganisms. The normal packaging and packing of food (to protect against moisture, dust, and bacterial or other contamination) provides protection against most biological agents. The exception may be toxins and biologically derived substances. However, the protective methods used for chemical agents will also protect against toxins and derived substances. Food in freezers, refrigerators, and in refrigerated trucks or rail cars will be safe if these containers remain sealed until the outer surfaces are decontaminated.
(1) It is unlikely that a biological agent will affect the appearance, taste, or smell of the food enough for the change to be apparent.
(2) Packaging and packing materials are not life supportive to pathogenic agents and are, therefore, self-decontaminating with the exception of spore-forming organisms.
(3) Most operational rations are packaged in metal containers, or encased in heavy aluminum laminated plastics that can withstand boiling water; also, they are impervious to arthropod penetration. This food is highly resistant to biological agents.
(4) The use of unpackaged items (unwrapped meats, fresh fruits, and vegetables) should be restricted; use only operational rations. Unprotected fresh food stored in the open and close to the source of dissemination will become contaminated.
b. Detection.
(1) Rapid identification of agents used is absolutely essential to implement effective countermeasures. Agent identification must be achieved quickly; it is the first step in answering critical management questions. What adjustments must be made in food preparation and distribution? What are the essential countermeasures? What is the expected outcome of the incident?
(2) Samples of food that are suspected of being contaminated are transported to the designated supporting laboratory. Samples must be accompanied by a description of the samples, the sample collection procedures, and the circumstances, which prompted the collection. The designated medical laboratory in the TO will provide a field confirmation identification of the agent(s). Designated CONUS laboratories accomplish definitive identification. See Appendix B for sampling procedures.
NOTE
New biological detection equipment is under development that will enable units to conduct presumptive identification of biological warfare agents. However, samples must also be collected and processed as described in Appendix B.
c. Decontamination.
(1) Food contaminated with toxins is handled in the same manner as food contaminated with chemical agents. Food contaminated with microorganisms is handled in the same manner as when contaminated with the more common foodborne disease-producing microorganisms.
(2) Several methods are available to decontaminate food items contaminated with biological agents. The following decontamination methods are considered to be the minimum. See Table J-1.
(3) Group II food that is sealed in containers that are resistant to the passage of biological agents requires only that the exterior of the container be decontaminated. Decontamination of these items is as follows:
(a) For containers made of metal, glass, plastic, or porcelain:
1. Thoroughly wash the container in potable water and soap, or in a disinfectant solution. If the water used for washing is contaminated, the soap and water wash may increase, not reduce, the contamination hazard. After which, the food containers are immersed in a disinfectant solution for 30 minutes (see Table J-3); then rinsed with potable water, if available and time permits. Chlorine solutions are not as reactive or corrosive as DS2.
2. Place the containers in boiling soapy water for 15 minutes; then rinse with potable water.
NOTES
1. The chemical field decontamination kits do not meet the requirements to decontaminate food supplies exposed to biological agents.
2. The same procedures should be followed even if there is only suspicion of a biological warfare attack.
(b) Thoroughly wipe containers that will not withstand soaking with a cloth soaked in a chlorine detergent solution. Remove the food from the container and place it in Group III.
(c) Metal or glass containers determined to have trichothecenes (Yellow Rain) present can be decontaminated using DS2. Allow a contact time of 5 to 30 minutes for the DS2 to neutralize the toxin. Then rinse the container with potable water.
(4) Group III food items that are not protected by the packaging material are decontaminated or disposed of as follows:
(a) Decontaminate foods that can be peeled or pared by immersing them in a disinfectant solution for 30 minutes, and then rinsing them with potable water (see Table J-3). Peel or pare the items after decontamination, then wash and, if appropriate, cook before eating.
(b) With the exception of certain heat-stable toxins, heat is the most practical means of decontaminating food. Several heating methods may be used, but the method chosen depends upon the type of food to be decontaminated. The key is to apply as much heat as possible without rendering the food unfit.
1. Cook in a pressure-type cooker with 15 pounds of pressure at 250°F (121°C) for 15 minutes.
2. Cook in a low-pressure cooker at 228°F (109°C) for 1 hour.
3. Bake bread or related items at 400°F (204°C) for 40 minutes.
CAUTION
Bread made with toxin-contaminated flour (especially with trichothecenes) is still toxic.
4. Bake or roast meat at 325°F (163°C) for 2 hours.
5. Boil for at least 15 minutes when no other method is available.
(c) Although decontamination methods are provided above, vegetables such as lettuce, broccoli, and cauliflower, or unwrapped meats that have been exposed to biological agents should not be eaten.
(d) Foods, such as butter, ice cream, and bread that will not withstand any of the above treatments must be destroyed.
(5) Established meat inspection procedures are followed when animals exposed to biological agents must be used for food. The meat must be thoroughly cooked.
Table J-3. Chlorine Solutions for Decontamination of Biological Warfare Agents
| CHLORINE SOURCE | MIXTURE TO PRODUCE 200 PPM SOLUTION OF AVAILABLE CHLORINE |
|---|---|
| HOUSEHOLD BLEACH | 1/2 GAL/25 GAL WATER |
| HIGH-TEST HYPOCHLORITE (CALCIUM HYPOCHLORITE) | 1/2 LB/25 GAL WATER |
| SUPERTROPICAL BLEACH | 1 LB/25 GAL WATER |
a. Contamination.
(1) Contamination of foodstuffs by a chemical agent may occur at any point on the battlefield. This contact may render the food unpalatable also. In many cases, decontamination is difficult, thus, emphasis must be placed on protection. Keep food supplies covered at all times. Take special precautions to protect food that is not packed in protective packages. Unprotected food, forage, and grain supplies may be so contaminated that their consumption will produce gastrointestinal irritation, or systemic poisoning. Nerve agents, vesicants, and arsenicals are the most dangerous. Field concentrations of phosgene, hydrocyanic acid, irritants, and smokes will seldom be high enough to cause serious food contamination. The effect of CK on food is not known. As a precaution, foods exposed to CK should be considered toxic.
(2) The effects of chemical agents on food depend on the nature of the agent and the type of the food. The extent to which chemical agents penetrate food also depends on the amount, form of dispersal (liquid [droplet size], or vapor) and duration of exposure. Nerve agents and mustard will penetrate deeply into unprotected fatty foods and will readily penetrate granular products such as grain and sugar. Liquid food products can be completely contaminated. Arsenicals readily hydrolyze to poisonous arsenical oxides in some foods. Foods can be divided into three categories based on their water content, fat content, and crystalline structure:
(a) Foods having a high water content, a low fat content, and/or a crystalline structure (fresh vegetables, fruits, sugar, salt, and eggs) will absorb mustard and nerve agents, either as a liquid or as a vapor. Nerve agents will be hydrolyzed slowly.
(b) Foods having a low fat content and an irregular (amorphous) structure (flour, bread, grain, rice, cereals, dried fruits, dried vegetables, tea, coffee, peas, and beans) readily absorb mustard and nerve agents in liquid form. As a vapor, these agents are absorbed to some extent, but are easily removed by airing.
(c) Foods having a low water content and a high fat content, such as butter, fat, fatty oils, ham, cheese, milk, bacon, fatty meat, and fish, absorb mustard and nerve agents such that removal of the agents is virtually impossible.
(3) Chemical agents can be physically and chemically absorbed into food. In addition to the toxic effect, they often adversely affect taste, smell, and the appearance of the food. However, chemical agents can cause the food to become very toxic without causing any other changes in the food. Table J-4 shows the effects of a number of chemical agents on food. Since food can be contaminated without any outward change in appearance, the possibility of contamination must be assumed in a chemical agent environment. Treat the food with the same precautions as established for known contaminated items.
(4) The protective properties of packaging materials are dependent upon a number of factors. The factors include the form of the agent (liquid versus vapor); concentration and exposure time; weather (temperature, wind speed, and humidity); and packaging material (the type of material, thickness, and the presence of folds, tears, and small holes). Even the thinnest material will offer some protection and is better than nothing at all. Therefore, always cover food supplies with whatever material is available. Table J-5 summarizes the protection values of various packaging materials against vapors and liquids.
(a) Operational rations are substantially protected while contained in the shipping cases and especially if stored in the original palletized unit load with an overlay of fiberboard, shrink wrap, or film wrap. The worst case is pallets of subsistence contaminated by liquid droplets during an attack. After the attack, high vapor concentrations will exist in the vicinity of the palletized loads. If the outer barrier is permeable such as fiberboard, it is possible that a liquid agent can seep through the overlay fiberboard and contact the shipping containers in liquid form. Normally, with seepage resistant materials, such as shrink wrap as the outer barriers, only the vapors of the agent are found within the pallet.
(b) While MREs are stored, the food is protected by up to six layers of material. Multilayer barriers result in a complex diffusion process of the agent from the outside towards the interior. Vapor penetration into nonhermetically sealed spaces is a simple gaseous diffusion process. Permeation through packaging is a much more complex process regardless of whether the challenge is a liquid or a vapor.
1. Liquid is adsorbed into permeable materials such as fiberboard or chipboard. With permeation-resistant materials (such as shrink wrap), the agent dissolves into, seeps through, and then desorbs from the barrier material. Shrink wrap provides adequate protection. Fiberboard sheathing provides adequate protection against mustard agents, but not against nerve agents.
2. The low-density polyethylene used to construct the menu bag can absorb chemical agents and possibly toxins. If the menu bag is removed from the shipping container and is exposed to liquid contamination, enough agent may pass through the bag to create a health hazard. Keep MREs in the shipping container until issued to the soldier. The menu bags should then be kept under the same degree of protection as the soldier.
3. The aluminum-laminated materials used to construct the MRE (retort and nonretort) pouches protect food from chemical contamination if hermetically sealed. The only item in the MRE meal bag that is not adequately protected is the spoon.
(5) Mylar and cellophane are resistant to chemical agents.
Table J-4. Effects of Chemical Agents on Food
| INFLUENCE ON | RESIDUAL TOXICITY | |||
|---|---|---|---|---|
| AGENT | TASTE | SMELL | COLOR | |
| MUSTARD | BAD | BAD | DISCOLORS MEAT | + |
| N-MUSTARDS | BAD | BAD | DOESN'T DISCOLOR MEAT | + |
| ARSENICALS | ACID | BAD | DISCOLORS MEAT AND VEGETABLES | +, ARSENIC |
| NERVE AGENTS | BAD | NONE | NONE | + |
| PHOSGENE | ACID | NONE | ? | - AFTER WEATHERING |
| CYANOGEN AGENTS | BITTER | BAD | NONE | - AFTER WEATHERING |
| IRRITANTS | ACID | BAD | NONE | + |
| SMOKE | ACID | BAD | ? | - |
| WHITE PHOSPHOROUS | ? | ? | ? | + |
| + INDICATES THE PRESENCE OF RESIDUAL TOXICITY. | ||||
| - DENOTES THAT RESIDUAL TOXICITY IS NOT PRESENT. | ||||
| ? THE INFLUENCE HAS NOT BEEN DETERMINED. | ||||
Table J-5. Protection from Chemical Contamination by Packaging Methods and Materials
| CHEMICAL VAPORS | LIQUIDS | |
|---|---|---|
| BOTTLES AND CANS | ||
| AIRTIGHT BOTTLES | COMPLETE | COMPLETE |
| SEALED METAL CANS | COMPLETE | COMPLETE |
| GLASS BOTTLES | GOOD | GOOD |
| METAL CONTAINERS | GOOD | GOOD |
| BOXES | ||
| CARDBOARD | MODERATE | MODERATE |
| WOODEN CRATES | MODERATE | POOR OR NONE |
| WRAPPINGS | ||
| METAL FOIL LAMINATES | COMPLETE | COMPLETE |
| PAPER | POOR | NONE |
| TEXTILES | NONE | NONE |
| WAXED PAPER | GOOD | MODERATE |
| MULTILAYER BAGS | GOOD | MODERATE |
| CELLOPHANE | GOOD | GOOD |
| CELLOPHANE, WET | NONE | NONE |
| CANVAS | POOR | POOR |
b. Detection.
(1) Currently, a field method for detecting chemical agent contamination in food does not exist. Contamination is not always spread evenly throughout food; this makes it impossible to take a single sample and determine the presence or absence of chemical agents in the entire lot. Additionally, standardized laboratory tests have not been developed for determining levels of chemical agents in food. Until a specific method to detect chemical agents in food is available, reliance will have to be made upon determination of contamination, or lack thereof, on the packaging material; the integrity of the packaging material; the protective qualities of the packaging material; and the penetration characteristics of the suspected chemical agents.
(2) Food may become toxic without any change in outward appearance. Never taste or smell food to determine if contamination is present in food.
(3) Veterinary and subsistence units have the following equipment available to detect chemical agents in the field:
(a) The M8 Automatic Chemical Agent Alarm System consists of the M43 detector unit and the M42 alarm unit. The detector unit is a portable, automatic, point-monitoring device that is designed to be hand carried from point to point. The M8 is used to provide early warning of a toxic agent position and detects the presence of chemical vapors and aerosols. The M43 detects all nerve, blood, and choking agents, and some blister agents. The M43A1 (the replacement for the M43) only detects nerve agents.
(b) The M256 Chemical Agent Detector Kit detects and identifies nerve, blood, and blister agents. The M256 is the most sensitive of the chemical agent vapor detectors available. However, it is not a continuous, real-time monitoring system. It requires 15 to 20 minutes for sampling and analysis.
(c) The ABC-M8 VGH Chemical Agent Detector Paper can detect and differentiate between nerve and blister agents by color change. It is intended to be used by blotting and wiping surfaces suspected of contamination. The M8 paper will respond with a visual color change in 10 seconds or less.
(d) The M9 Chemical Agent Detector Paper will detect liquid nerve (G & V) and blister agents (H & L), but will not identify the specific agent or differentiate between nerve and blister agents. The M9 tape is sensitive to droplets as small as 100 μ, and will respond with a visual color change in 10 seconds or less.
(4) All subsistence in a chemical attack area are considered contaminated until a survey can be conducted, preferably by veterinary and chemical personnel. Personnel must be at MOPP Level 4 while conducting the survey. Concentrate the initial portion of the survey on the adequacy of the storage facility and other protective measures in preventing chemical agent contact with subsistence items. The area surrounding the storage facility is examined for the presence of animals, rodents, birds, and arthropods acting unusual, or dead in unusual numbers. If animals are present and assistance is required in identifying the NBC agent, specimens can be collected and submitted to the area medical laboratory. Damage such as broken windows, holes, or loss of structural integrity of the storage facility is noted. This information combined with knowledge of the agent form (liquid or vapor), type of agent (which will indicate the degree [J-17] of persistency), and approximate time of attack will provide a risk assessment. Liquid agents should not significantly penetrate an intact facility, but may produce vapor contamination by off-gassing.
(a) Upon entering the storage facility, the M8 can be used to determine the presence of chemical vapors. However, precautions must be taken. The M42 alarm is not to be used inside shelters, vehicles, vans, or other interior modes. Therefore, when checking food storage facilities, the alarm unit must be left outside, turned off, or disconnected. Do not tilt the M43 detector more than 45 degrees (because of the liquids it contains). This is not a problem with the improved M43A1, but the M43A1 requires attachment of an exit port filter when used indoors. The M256 Chemical Agent Detector Kit can be used to sample the air.
(b) Pre-position M9 chemical agent detector paper in food storage areas; especially on the least protected pallets and in areas where droplets may enter, such as near doors or windows. Examine the M9 paper for indications of liquid chemical agents. If the M9 paper is positive, or if the packaging materials show the presence of liquids or stains, use the M8 detector paper to determine the type of the agent. If an agent is not indicated by the detector paper, then the amount of agent present will be insufficient to cause secondary contamination when the outer package is removed.
(5) Detection procedures become more complicated if a chemical agent has penetrated or permeated through the packaging and packing materials. Unless liquid has seeped through the cardboard, any agent in the interior of the shipping case will be in a vapor form. Liquid seeping should be obvious. The sampler-detectors in the M256 Chemical Agent Detector Kit do not have an aspirator for sampling the interior of the case. However, there are several procedures that can be used. One is to open the case, place the activated sampler-detectors inside the case, and then reclose the case. Another is to punch holes in the case, place the activated sampler-detector over the holes, and cover the sampler-detector with an empty box or can (open end down) to concentrate the vapors escaping from the case. Alternatively, remove the food from the case and place it in a plastic bag with the sampler-detectors to concentrate the vapors. These procedures require two sampler-detectors; one for blood agents and one for nerve and blister agents. Neither method is very sensitive in low concentrations of vapor as is expected to be present inside shipping containers. A better method is to modify the M43 detector with a field expedient probe of Teflon tubing attached to the detector's air inlet. Insert the open end of the tubing into a hole in the case or package to sample the interior air. When available, the improved chemical agent monitor (ICAM) can be used; its design will allow aspiration of air from inside shipping cases. The ICAM can also be used to detect and identify liquid agents on a surface provided the agent is vaporizing in sufficient quantity. The ICAM gives a visual representation of a hazard evaluation.
c. Decontamination.
(1) Decontamination is only required for contamination remaining 10 minutes or longer. Decontamination efforts on subsistence items will normally be limited to removal of the containers and carton overwrap material.
(2) The need for decontamination is primarily dictated by the type of chemical agent used. The method of decontamination selected will depend upon the type of packaging material used and the urgency with which the food is required.
(3) Food supplies in storage are not likely to be seriously contaminated if reasonable protection precautions are taken. For this reason, large supplies of food are not to be condemned as a whole simply because they have been exposed to possible chemical contamination. A prompt and careful survey of the supplies may reveal that only a few items have been contaminated to a level that decontamination is required. Prompt segregation of the heavily contaminated portions will prevent, or minimize, contamination of the remainder. Foods without protective packages constitute the major difficulty.
(4) Individual decontamination is performed by each soldier on those subsistence items in his possession at the time of the attack. Individual decontamination is limited to operational rations that are in original, intact containers. Unit-level decontamination is performed by unit personnel under the supervision of unit NBC personnel. Support decontamination is attempted at major subsistence storage facilities. Again, decontamination is limited to packing material. Decontamination of food itself is only attempted in emergency situations when alternative supplies are not available.
(5) Start decontamination operations with the easiest method and proceed to the most difficult. This allows for the removal of a relatively large portion of the contamination in a minimum of time. The simplest procedure is to allow the materials to age and air ("weather"). Substantial self-decontamination will occur with most agents. Exceptions are thickened mustard, thickened GD, and VX. Table J-6 provides the length of time for which contaminated subsistence supplies may present a contact hazard. Weather elements that affect decontamination are—
(a) Warm temperatures speed liquid agent off-gassing and hasten the dispersion of chemical agents into the air.
(b) High winds rapidly disperse chemical agent vapors and speed off-gassing from surfaces.
(c) Moisture causes chemical agents to react with water to form nontoxic or less toxic chemicals. Heavy rain or rain of long duration can aid decontamination by mechanically removing chemical agents.
(d) Even in cold weather, direct sunrays warm surfaces above the air temperature and hasten the off-gassing and decomposition of chemical agents.
Table J-6. Persistency of Selected Liquid Chemical Agents
| WEATHER CONDITIONS | |||
|---|---|---|---|
| AGENT | SUNNY, AROUND 20°C, LIGHT BREEZE | WET AND WINDY, AROUND 10°C | CALM, SUNNY, LYING SNOW, AROUND -10°C |
| MUSTARD(HD) | 2-7 DAYS | 1/2-2 DAYS | 2-8 WEEKS |
| TABUN(GA) | 1-4 DAYS | 1/2-6 HOURS | 1 DAY-2 WEEKS |
| SARIN(GB) | 1/4-4 HOURS | 1/4-1 HOUR | 1-2 DAYS |
| SOMAN(GD) | 2-1/2-5 DAYS | 3-36 HOURS | 1-6 WEEKS |
| NERVE(VX) | 3-21 DAYS | 1-12 DAYS | 1-16 WEEKS |
(6) Active decontamination is attempted only when weathering will not decontaminate the packaging material in sufficient time. Decontamination procedures can be enhanced by using heat to vaporize the chemical agent; by reaction with decontaminants; or by removing with hot soapy water.
(a) The simplest (standard) decontamination materials are water and detergents. An effective decontaminant is hot water used with the addition of soap or detergent and scrubbing. Commercial abrasive powdered cleansers are effective decontaminants for many surfaces (metal, glass, Formica), but not wood or soft plastics.
(b) Water can be used to flush chemical agents from surfaces. High-pressure application produces a better cleansing action than low pressure. If the surface has absorbed the agent, flushing will remove the surface contamination, but will not affect the agent that is absorbed.
(c) Soaking contaminated items in boiling water is an excellent decontamination method for some agents. Water alone will not be sufficient to decontaminate all chemical agents. Soaking in warm or cold water may reduce the contamination slightly; however, the hazard may not be reduced sufficiently even after prolonged soaking. If hot water is not available, or if it might cause damage to the item, other methods of decontamination should be considered, such as decontaminating solutions or a caustic solution followed by thorough rinsing.
(d) Fibrous materials such as cloth and canvas are best decontaminated by washing and scrubbing.
(e) Glass, metal, porcelain, and plastic surfaces are best decontaminated by using hot water or hot soapy water. Some toxic materials are readily removed with no more than slight abrasion or brushing.
(f) Painted, varnished, and waxed surfaces are generally smooth and nonporous. Dust and liquids are readily removed by wiping, brushing, or vacuuming. Absorbed materials are removed by hot water, detergent, or complexing agents. None of these surfaces stand up well to heavy abrasive techniques. Agents can be attacked and removed by caustics, acids, and organic chemicals. Some of these surfaces readily absorb agents, so weathering following decontamination is advisable.
(g) Rubber is a porous material that can absorb agents. It is not easily decontaminated by abrasive techniques. Warm, soapy water used with brushing is effective since it removes some absorbed contamination. Strong acids, alkalies, and organic solvents may deteriorate and decompose rubber articles.
(7) Operational rations are the primary rations issued; always issue uncontaminated stocks first. This allows for decontamination of contaminated stocks without interrupting supply support. Normally, contaminated stocks are not issued. The decision to issue contaminated items is based on the tactical situation, criticality of the items, type and extent of contamination, and the time and resources available for decontamination. Decontamination efforts on subsistence items are limited to the containers and carton overwrap material.
(a) The MRE retort and nonretort food pouch may be decontaminated with soap and water wash. The chemical agents will be removed by the solutions.
(b) Semipermeable materials (polyethylene menu bag, shrink wrap, and film wrap) may have chemicals deposited not only on the surface, but also dissolved into the matrix of the material. The chemicals can be removed from the surface by washing with hot soapy water, but contaminant dissolved in the material is not removed. The remaining agent can only be removed by weathering which can be accelerated through the use of heat and sweeping the surface with air.
(c) Fiberboard is both sorbent and permeable and acts like a blotter. Liquid decontaminants can force the contaminant further into the fiberboard. Any attempt to decontaminate fiberboard would be futile. The only alternatives are to remove the fiberboard, or to allow it to weather.
(d) Palletized unit loads of MRE and UGR outerwraps can be decontaminated through the aid of a forced clean air sweep in 4 to 5 days, compared to 3 weeks or more under natural conditions without a forced air sweep.
(8) Contaminated food supplies are only handled by personnel trained in decontamination methods and in MOPP Level 4. Contaminated food items are divided into three groups as described below (see Table J-1 for additional information).
(a) Group 1 consists of canned and unopened packaged items which have been exposed only to agent vapors. Most items in this group will be safe to issue after a brief period of outdoor airing to remove clinging vapors. Table J-7 lists the decontamination procedures for packaging materials contaminated with nerve agents, mustards, and arsenicals.
Table J-7. Chemical Decontamination of Packaged Material
| PACKAGING MATERIAL | CONTAMINATION | DECONTAMINATION PROCEDURES |
|---|---|---|
| AIRTIGHT METAL | VAPOR AND | AIR FOR 24 HOURS. WASH |
| CONTAINERS, GLASS | LIQUID | WITH HOT SOAPY WATER, |
| BOTTLES, FOIL | SODA, OR BLEACH SOLUTION. | |
| ALUMINATED LAMINATED | RINSE WITH WATER. | |
| MATERIALS. | ||
| POLYESTER, PVF. | VAPOR | REMOVE CONTAMINATED |
| WOODEN BOXES, CRATES, | PACKAGE. AIR CONTENTS FOR | |
| BOARD, MULTILAYER | 24 HOURS. | |
| BAGS. | ||
| CARDBOARD, | LIQUID | CONTAMINATED CONTENTS— |
| POLYETHYLENE. | TREAT AS UNPACKAGED FOOD. | |
(b) Group II consists of canned and unopened packaged items which have been contaminated with a liquid chemical agent.
1. Attempts to decontaminate porous packaging materials, such as cardboard or wood, are likely to be unsuccessful and may result in spreading the contamination. The best procedure in [J-21] handling such items is to strip off the outer contaminated coverings and examine the inner layer to see if penetration of the agent has occurred. If it has, continue stripping off layers until an uncontaminated layer is reached and place it in Group I. If the agent has penetrated to the food, place it in Group III.
2. Food in cans or in other sealed, impermeable containers is not in danger of chemical contamination. Because contamination is confined to the outer surface of the sealed container, decontamination is accomplished by: immersion in boiling, soapy water for 30 minutes and rinse; immersion in boiling water for 30 minutes; spray with DS2; or to wash in hot soapy water, rinse, and aerate. Under no conditions should contaminated containers be opened before they have been decontaminated and monitored.
3. Supertropical bleach and DS2 can be used on the polyethylene menu bag for up to 24 hours without a significant change in appearance, tensile properties, and size of the plastic. The use of DS2 will cause significant degradative changes to most other plastics, while STB will cause little or no change. Also, DS2 may cause false positive readings when using M8 or M9 paper, or the M256 Detector Kit to check completeness of decontamination.
(c) Group III will consist of unpackaged or poorly packaged items which have been exposed to an agent in either vapor or liquid form. Foodstuffs in this group should be decontaminated only when absolutely necessary. The decision to use foods that have been contaminated is to be made by the commander. Decontamination procedure to be followed, in order, is: trim surface fat and grossly contaminated areas; wash with water or 2-percent sodium bicarbonate solution; then boil in water.
1. Boiling in water may be eliminated when the contamination has been only with the vapors of irritant agents. When such an exposure has been light, aeration for a short time may be used for decontamination.
2. Frying, roasting, or broiling will not remove traces of blister agents from meats. In general, salvage of foods heavily contaminated with droplets of the blister agents, especially the arsenical blister agents, is not practical. Foods of high water or fat content are unfit for consumption and reclamation is not practical when contaminated with liquid mustard or a liquid nitrogen mustard.
3. When foods have been exposed to blister agent vapor, they can be reclaimed by washing with sodium bicarbonate solutions and rinsing with clear water, by intensive cooking, or in the case of dry provisions, by 24 to 48 hours of aeration. Lean meat contaminated with mustard vapor can be reclaimed by boiling in water for 30 minutes or more. With nitrogen mustard vapor contamination, the meat should be boiled in a 2-percent sodium bicarbonate solution. Discard the water used to boil the meat.
4. Nerve agent contamination is treated the same as blister agent contamination.
5. Foods, such as potatoes and hard-skinned fruits and vegetables, can be decontaminated by washing or scrubbing, followed by peeling or scraping, then washing again.
6. Prepared food in open containers will be contaminated; it must be temporarily isolated, or disposed of (bury or as directed by commander).
7. A food Item that is contaminated with irritants can be decontaminated by airing. Consumability is determined by taste rather than toxicity.
8. Phosgene is rapidly hydrolyzed, therefore, washing the food with water or airing it will usually suffice.
9. Food contaminated with white phosphorous should be destroyed.
10. Normally, hydrocyanic acid will have little effect on food supplies. The exposures will most likely be as a vapor. However, foods with a high water content may become unfit for consumption after exposure to high concentrations.
11. The effect of CK on foods is not known. Foods exposed to CK vapors are considered toxic.
12. Table J-8 lists the decontamination procedures for unpackaged food contaminated with a chemical agent.
(9) Decontaminating cattle, poultry, and other livestock is only attempted when other sources of food are not available. Heavily contaminated animals should be destroyed. Livestock contaminated lightly by phosgene, nerve agents, mustards, and arsenicals (such as vapor or liquid) may be slaughtered in the early stages of poisoning before the full effects of exposure are shown. If these animals are slaughtered in the preliminary stages of poisoning and all tissues exposed to the agent (the head, blood, lungs, organs, and local areas) are discarded, there is no danger in consumption of the meat, provided the animal passes a pre-slaughter and slaughter inspection. This is true even of animals poisoned by arsenical agents since the edible tissue will contain amounts of arsenic too small to be toxic. Organs (liver, brain, heart, kidney, and lungs) will contain more arsenic than the musculature and are discarded. The meat must be well cooked. Personnel involved in slaughtering procedures must be careful to prevent spreading contamination to the meat and to themselves.
(10) Decontaminating forage and grain exposed to only chemical agent vapors is by aeration. Aerated supplies, especially if mixed with larger amounts of uncontaminated supplies, produces no ill effects when fed to animals. Forage or grain heavily contaminated by liquid vesicants, especially arsenicals, should not be used.
Table J-8. Chemical Decontamination of Unpackaged Food
| CHEMICAL AGENT | FATTY FOODS (BUTTER, BACON, MILK, CHEESE, HAM). | NONFATTY FOODS, HIGH WATER CONTENT CRYSTALLINE (FRUITS, VEGETABLES, SALT, SUGAR). | NONFATTY FOODS, LOW WATER CONTENT, AMORPHOUS (FLOUR, CEREALS, BREAD, PEAS). |
|---|---|---|---|
| NERVE AGENTS | |||
| VAPOR, HEAVY | DESTROY | DESTROY, UNLESS POSSIBLE TO BOIL AFTER AIRING 48 HOURS. | AIR FOR 48 HOURS, THEN BOIL. |
| [J-23] VAPOR, LIGHT | DESTROY | AIR FOR 48 HOURS, THEN BOIL. | AIR FOR 48 HOURS, THEN BOIL. |
| LIQUID | DESTROY | DESTROY | DESTROY |
| MUSTARDS | |||
| VAPOR | REMOVE 1-3 cm OF OUTER LAYER AND WASH WITH 2% SODIUM BICARBONATE SOLUTION. BOIL FOR AT LEAST 30 MINUTES. DESTROY MILK. | WASH WITH WATER. AIR FOR 48 HOURS. | WASH WITH WATER. AIR FOR 48 HOURS. |
| LIQUID | DESTROY | DESTROY | DESTROY |
| ARSENICALS | |||
| DESTROY | DESTROY | DESTROY | |
ABBREVIATION, ACRONYMS, AND DEFINITIONS
NATO
NATO Emergency War Surgery Handbook. 1988.
NATO STANAGs
These agreements are available on request (using DD Form 1425) from the Standardization Documents Order Desk, 700 Robins Avenue, Building 4, Section D, Philadelphia, Pennsylvania 19111-5094.
2002. Warning Signs for the Marking of Contaminated or Dangerous Land Areas, Complete Equipments, Supplies and Stores. Edition 8. 29 January 1999.
2047. Emergency Alarms of Hazard or Attack (NBC and Air Attack Only). Edition 7. 24 July 1998. (Latest Amendment, 7 February 2000.)
2068. Emergency War Surgery. Edition 4. 28 October 1986. (Latest Amendment, 17 October 1991.)
2083. Commander's Guide on Nuclear Radiation Exposure of Groups. Edition 5. 19 September 1986. (Latest Amendment, 26 June 1994.)
2103. Reporting Nuclear Detonations, Biological and Chemical Attacks, and Predicting and Warning of Associated Hazards and Hazard Areas—ATP 45(A). Edition 8. 31 August 2000.
2104. Friendly Nuclear Strike Warning. Edition 7. 28 June 1994. (Latest Amendment, 28 June 1995.)
2112. Nuclear, Biological, and Chemical Reconnaissance. Edition 4. 6 March 1998.
2475. Medical Planning Guide for the Estimation of NBC Battle Casualties (Nuclear)—AmedP-8(A), Volume I. December 2000.
2476. Medical Planning Guide of NBC Battle Casualties (Biological)—AmedP-8(A), Volume II. March 2001.
2477. Planning Guide for the Estimation of NBC Battle Casualties (Chemical)—AmedP-8(A), Volume III. March 2001.
2500. NATO Handbook on the Medical Aspects of NBC Defensive Operations—AMedP-6(B). Edition 4. 11 February 1997.
2873. Concept of Operations of Medical Support in Nuclear, Biological, and Chemical Environments—AmedP-7(A). Edition 3. 16 October 1996.
2879. Principles of Medical Policy in the Management of a Mass Casualty Situation. Edition 3. 7 September 1998.
2941. Guidelines for Air and Ground Personnel Using Fixed and Transportable Collective Protection Facilities on Land. Edition 2. 19 June 1992. (Latest Amendment, 30 October 1995.)
2954. Training of Medical Personnel for NBC Operations. Edition 1. 28 December 1987. (Latest Amendment, 6 June 1995.)
ABCA QSTAGs
These agreements are available on request (using DD Form 1425) from the Standardization Documents Order Desk, 700 Robins Avenue, Building 4, Section D, Philadelphia, Pennsylvania 19111-5094.
183. Emergency Warning Signals and Alarms for NBCD Hazards or Attacks (NBC and Air Attacks Only). [RF-2]Edition 3. 12 August 1991.
187. Reporting Nuclear Detonations Biological and Chemical Attacks and Predicting and Warning of Associated Hazards and Hazard Areas. Edition 5. 21 May 1998.
189. Friendly Nuclear Strike Warning. Edition 3. 12 August 1991.
501. Warning Signs for the Marking of Contaminated or Dangerous Land Areas, Complete Equipment, Supplies and Stores. Edition 2. 11 May 1982.
608. Interoperable Chemical Agent Detector Kits. Edition 3. 13 December 2000.
816. Medical Aspects of Mass Casualty Situations. Edition 1. August 1990.
1330. Medical Aspects of NBC Defensive Operations. Draft.
2000. Guidelines on Entry and Exit Procedures for Using Collective Protection Facilities. 17 December 1996.
JOINT OR MULTISERVICE PUBLICATIONS
AR 40-535. Worldwide Aeromedical Evacuation. AFR 164-5; OPNAVINST 4630.9C; MCO P4630.9A. 1 December 1975. (Reprinted with basic including Change 1, 10 May 1979.)
AR 40-562. Immunizations and Chemoprophylaxis. AFJI 48-110; BUMEDINST 6230.15; CG COMDTINST M6230.4E. 1 November 1995.
AR 40-656. Veterinary Surveillance Inspection of Subsistence. NAVSUPINST 4355.10; MCO 10110.45. 15 October 1986.
AR 40-657. Veterinary/Medical Food Inspection and Laboratory Service. NAVSUPINST 4355.4F; MCO P10110.31G. 6 November 1997.
FM 3-3. Chemical and Biological Contamination Avoidance. FMFM 11-17. 16 November 1992. (Change 1, 29 September 1994.)
FM 3-4. NBC Protection. FMFM 11-9. 29 May 1992. (Reprinted with basic including Changes 1-2, 21 February 1996.)
FM 3-5. NBC Decontamination. MCWP 3-37.3. 28 July 2000.
FM 3-6. Field Behavior of NBC Agents (Including Smoke and Incendiaries). AMF 105-7; FMFM 7-11 H. 3 November 1986.
FM 3-9. Potential Military Chemical/Biological Agents and Compounds. NAVFAC P-467, AFR 355-7. 12 December 1990.
FM 3-11.34. Multiservice Procedures for Nuclear, Biological, and Chemical (NBC) Defense of Theater Fixed Sites, Ports, and Airfields. MCWP 3.37.5; NTTP 3-11.23; AFTTP(I) 3-2.33. 29 September 2000.
FM 3-19. Nuclear, Biological and Chemical Reconnaissance. FMFM 11-20. 19 November 1993.
FM 3-100. Chemical Operations, Principles and Fundamentals. MCWP 3-3.7.1. 8 May 1996.
FM 4-02.33. Control of Communicable Diseases Manual. 17th Edition. NAVMED P-5038. 31 December 1999.
FM 4-02.283. Treatment of Nuclear and Radiological Casualties. NTRP 4-02.21; AFMAN 44-161(I); MCRP 4-11.1B. 20 December 2001.
FM 6-22.5. Combat Stress. MCRP 6-11C; NTTP 1-15M. 23 June 2000.
FM 8-9. NATO Handbook on the Medical Aspects of NBC Defensive Operations AMedP-6(B), Part I—Nuclear, Part II—Biological, Part III—Chemical. NAVMED P-5059; AFJMAN 44-151V1V2V3. [RF-3]1 February 1996.
FM 8-284. Treatment of Biological Warfare Agent Casualties. NAVMED P-5042; AFMAN(I) 44-156; MCRP 4-11.1C. 17 July 2000. (Change 1, 8 July 2002.)
FM 8-285. Treatment of Chemical Agent Casualties and Conventional Military Chemical Injuries. NAVMED P-5041; AFJMAN 44-149; FMFM 11-11. 22 December 1995.
FM 21-10. Field Hygiene and Sanitation. MCRP 4-11.1D. 21 June 2000.
DEPARTMENT OF DEFENSE
DOD 5100.52-M. Nuclear Weapon Accident Response Procedures (NARP). September 1990.
DOD Directive 3150.8. DOD Response to Radiological Accidents. 13 June 1996.
DD Form 1380. US Field Medical Card. December 1991.
DD Form 1911. Material Courier Receipt. May 1982.
DEPARTMENT OF THE ARMY FORM
DA Form 4137. Evidence/Property Custody Document. 1 July 1976.
ARMY PUBLICATIONS
AR 40-5. Preventive Medicine. 15 October 1990.
AR 40-61. Medical Logistics Policies and Procedures. 25 January 1995.
AR 40-66. Medical Records Administration and Health Care Documentation. 3 May 1999.
AR 40-400. Patient Administration. 12 March 2001.
FM 3-06.11. Combined Arms Operations in Urban Terrain. 28 February 2002.
FM 3-7. NBC Field Handbook. 29 September 1994.
FM 3-50. Smoke Operations. 4 December 1990. (Change 1, 11 September 1996).
FM 3-101. Chemical Staffs and Units. 19 November 1993.
FM 3-101-4. Biological Detection Platoon Operations—Tactics, Techniques, and Procedures. 9 June 1997. (Reprinted with basic including Changes 1-2, 1 September 2000.)
FM 4-02.1. Combat Health Logistics. 28 September 2001.
FM 4-02.4. Medical Platoon Leaders' Handbook—Tactics, Techniques, and Procedures. 24 August 2001.
FM 4-02.6. The Medical Company—Tactics, Techniques, and Procedures. 1 August 2002.
FM 4-02.10. Theater Hospitalization. 29 December 2000.
FM 4-02.17. Preventive Medicine Services. 28 August 2000.
FM 4-02.19. Dental Service Support in a Theater of Operations. 1 March 2001.
FM 4-25.12. Unit Field Sanitation Team. 25 January 2002.
FM 8-10. Health Service Support in a Theater of Operations. 1 March 1991.
FM 8-10-1. The Medical Company—Tactics, Techniques, and Procedures. 29 December 1994.
FM 8-10-6. Medical Evacuation in a Theater of Operations—Tactics, Techniques, and Procedures. 14 April 2000.
[RF-4] FM 8-10-8. Medical Intelligence in a Theater of Operations. 7 July 1989.
FM 8-10-9. Combat Health Logistics in a Theater of Operations—Tactics, Techniques, and Procedures. 3 October 1995.
FM 8-10-14. Employment of the Combat Support Hospital—Tactics, Techniques, and Procedures. 29 December 1994.
FM 8-10-15. Employment of the Field and General Hospitals—Tactics, Techniques, and Procedures. 26 March 1997.
FM 8-10-18. Veterinary Service—Tactics, Techniques, and Procedures. 22 August 1997.
FM 8-10-24. Area Support Medical Battalion—Tactics, Techniques, and Procedures. 13 October 1993.
FM 8-10-25. Employment of Forward Surgical Teams—Tactics, Techniques, and Procedures. 30 September 1997.
FM 8-10-26. Employment of the Medical Company (Air Ambulance). 16 February 1999. (Change 1, 20 May 2002.)
FM 8-30. Veterinary Food Inspection Specialist. 12 August 1986.
FM 8-42. Combat Health Support in Stability Operations and Support Operations. 27 October 1997.
FM 8-50. Prevention and Medical Management of Laser Injuries. 8 August 1990.
FM 8-51. Combat Stress Control in a Theater of Operations—Tactics, Techniques, and Procedures. 29 September 1994.
FM 8-55. Planning for Health Service Support. 9 September 1994.
FM 8-250. Preventive Medicine Specialist. 27 January 1986. (Reprinted with basic including Change 1, 12 September 1986.)
FM 8-500. Hazardous Materials Injuries: A Manual for Pre-Hospital Care. Edition 4. 17 January 1997.
FM 10-52. Water Supply in Theaters of Operations. 11 July 1990.
FM 21-11. First Aid for Soldiers. 27 October 1988. (Reprinted with basic including Changes 1-2, 4 December 1991.)
FM 22-51. Leaders' Manual for Combat Stress Control. 29 September 1994.
FM 31-71. Northern Operations. 21 June 1971.
FM 90-3. Desert Operations. FMFM 7-27. 24 August 1993.
FM 90-5. Jungle Operations. 16 August 1982.
FM 90-10. Military Operations on Urbanized Terrain (MOUT). 15 August 1979.
STP 8-91 W15-SM-TG. Soldier's Manual, Skill Levels 1/2/3/4/5 and Trainer's Guide, MOS 91W, Health Care Specialist. 10 October 2001.
TC 8-13. Deployable Medical Systems—Tactics, Techniques, and Procedures. 7 December 1990.
TM 3-4240-288-12&P. Operator's and Unit Maintenance Manual including Repair Parts and Special Tool List for Collective Protection Equipment NBC, Simplified M20 (NSN 4240-01-166-2254). NAVFAC P-475. 20 August 1987. (Reprinted with basic including Changes 1-2, 3 May 1989.)
TM 10-5410-228-10. Operator's Manual for Chemical Biological Protective Shelter System. 31 August 2001.
TM 10-5410-283-14&P. Operator's Unit, Direct Support, and General Support Maintenance Manual (Including Repair Parts and Special Tools Lists) for Chemically Protected Deployable Medical System (CP DEPMEDS) (NSN 5410-01-479-9730) (5410-01-479-9727) and CP DEPMEDS Training Set (6910-01-479-2464). 30 November 2001.
References are to paragraph numbers unless otherwise indicated.
FM 4-02.7 (FM 8-10-7)
1 OCTOBER 2002
By Order of the Secretary of the Army:
ERIC K. SHINSEKI
General, United States Army
Chief of Staff
Official:
Administrative Assistant to the
Secretary of the Army
0225424
DISTRIBUTION:
Active Army, U.S. Army Reserve and Army National Guard: To be distributed in accordance with the initial distribution Number, 114899, requirements for FM 4-02.7.
TRANSCRIBER'S NOTE
Blank space in some sample documents in the text is denoted by ____.
Footnotes are all within a specific Table, including the Table of Contents. They are positioned at the bottom of that Table, as in the original text, and are denoted by [*] or [**].
Page numbering of the original text has been retained. It is in the form a-b, where a is a Chapter number or Appendix letter, and b is the sequential number within that section. For example B-3 is the third page in Appendix B.
Obvious punctuation errors have been corrected after careful comparison with other occurrences within the text and consultation of external sources.
Fractions in the original text are mostly full-height format, eg 1/2, and have been left in that form. Some are half-height and have been converted to Unicode fractions eg ½ ¾ whenever possible.
Except for those changes noted below, all misspelling in the text, and inconsistent or archaic usage, have been retained.
Chapter-Paragraph:
2-1. 'thermoberic' replaced by 'thermobaric'.
3-3. 'proves' replaced by 'provides'.
3-8. 'company's' replaced by 'companies'.
4-3. 'are also' replaced by 'is also'.
Appendix:
Table A-1. 'RADIATON' replaced by 'RADIATION'.
Table A-7. 'Cal/cm2/sec' replaced by 'Cal/cm2/sec'.
Table A-10. '0.8%-2% CANCER.' replaced by '0.8%-2%'.
Table A-13. 'Characteristics' replaced by 'Characteristics of'.
Para B-11. 'handsful' replaced by 'handful'.
Para B-18. 'BLUFFED VISION' replaced by 'BLURRED VISION'.
Para J-5. 'modify to M43' replaced by 'modify the M43'.
Index:
heat injury prevention. '6-10g(4)' replaced by 'G-8'.
monitor for completeness. '6-10f' replaced by 'G-10g'.