
PRESIDENT G. K. GILBERT,
American Association for the Advancement of Science.
Transcriber’s note: Table of Contents added by Transcriber.
EDITED BY
J. McKEEN CATTELL
VOL. LVII
MAY TO OCTOBER, 1900
NEW YORK AND LONDON
McCLURE, PHILLIPS AND COMPANY
1900
Copyright, 1900,
By McCLURE, PHILLIPS AND COMPANY.

PRESIDENT G. K. GILBERT,
American Association for the Advancement of Science.
JULY, 1900.
It would be difficult to name any subject of investigation, the progress of which during our time has been more remarkable than that in the field of stellar astronomy. Several features of this progress are especially noteworthy. One of these is the mere extension of research. A natural result of the northern hemisphere being the home of civilized peoples was that, thirty years ago, the study of the southern heavens had been comparatively neglected. It is true that the curiosity of the inquiring astronomers of the past would not be satisfied without their knowing something of what was to be seen south of the equator. Various enterprises and establishments had therefore contributed to our knowledge of the region in question. As far back as 1667, during a voyage to St. Helena, Halley catalogued the brighter stars in the region near the South Pole. About 1750 Lacaille, of France, established an observing station at the Cape of Good Hope, and made a catalogue of several thousand stars which has remained a handy book for the astronomer up to the present time. In 1834–38 Sir John Herschel made a special voyage to the Cape of Good Hope, armed with the best telescopes which the genius of his father had shown him how to construct, for the purpose of doing for the southern heavens as much as possible of what his father had done for the northern. The work of this expedition forms one of the most important and interesting chapters in the history of astronomic science. Not only is Herschel’s magnificent volume a classic of astronomy, but the observations which it contains are still as carefully and profitably studied as any that have228 since been made. They may be said to form the basis of our present knowledge of the region which they included in their scope.
Herschel’s work may be described as principally in the nature of an exploration. He had no instruments for accurately determining the positions of stars. In the latter field the first important contributions after Lacaille were made by Sir Thomas Brisbane, Governor of New South Wales, and Rumker, his assistant, at Paramata. Johnson, of England, about 1830, introduced modern accuracy into the construction of a rather limited catalogue of stars which he observed at St. Helena. About the same time the British Government established the observatory at the Cape of Good Hope, which has maintained its activity to the present time, though, at first, its means were extremely limited. About the middle of the century the Government of New South Wales established, first at Williamstown and then at Melbourne, an observatory which has worked in the same field with marked success.
An American enterprise in the same direction was that of Captain James M. Gilliss, who, in 1849, organized an astronomical expedition to Chili. The principal motive of this enterprise was the determining of the solar parallax by observations upon Venus and Mars near the time of their nearest approach to the earth. As these observations would take but a small part of his time, Gilliss determined to take with him instruments for determining the positions of the stars. He established his observatory at a point near Santiago, where he continued his observations for nearly three years. He was a practical observer, but an untoward circumstance detracted from the value of his work. His observatory was built upon a rocky eminence, a foundation which seemed to afford the best possible guarantee of the stability of his instruments. He made no attempt to reduce his observations till after his return home. Then it was found that the foundation, through the expansion and contraction due to the heat of the sun, was subject to a diurnal change which made it extremely difficult to derive good results from his careful work. It was not until 1896, more than thirty years after his death, that the catalogue of the stars observed by him was at last completed and published.
We do not derogate in any way from the merit of these efforts in saying that they could not lead to results comparable with those of the score of richly equipped northern observatories which the leading nations and universities of Europe had endowed and supported for more than a hundred years. Only within the last thirty years has it been possible to bring our knowledge of the southern heavens up to a satisfactory stage. Now, however, the progress of southern astronomy, if we may use the term, is such that in several points our knowledge of the southern heavens surpasses that of the northern ones. If we measure institutions by the importance of the work they are doing,229 there are several in the southern hemisphere which must to-day be placed in the first rank.
The history and work of the Cordova Observatory are of special interest. In 1870 Dr. B. A. Gould, who might fairly be considered as the father of modern American astronomy, conceived the idea of establishing an observatory of the first class in South America. He found the President and Governor of the Argentine Republic ready to support his scheme with a liberality well fitted to impress us with a high sense of their standard of civilization. In a year or two the observatory at Cordova was in active operation. A statement of its work belongs to a subsequent chapter. Suffice it to remark here that Dr. Gould continued in active charge until 1885, when he returned home, and was succeeded by Thome, the present director.
A few years after Gould went to Cordova, Gill was made director of the Royal Observatory at the Cape of Good Hope. The rapid growth of this institution to one of the first rank is due no less to the scientific ability of the new director than to the unflagging energy which he has devoted to the enlargement of the resources of the institution. The great fact which he sought to impress upon his supporters was that the southern celestial hemisphere was as large as the northern, and therefore equally worthy of study.
In any general review of the progress of stellar astronomy during the past twenty years, we should find Harvard University before us at every turn. What it has done will be seen, perhaps in an imperfect way, in subsequent chapters. Not satisfied with the northern hemisphere, it has established a branch at Arequipa, Peru, in which its methods of observation and research are extended to the south celestial pole. Its principal specialties have been the continuous exploration of the heavens. Celestial photography, photometry and spectroscopy sum up its fields of activity. For more than ten years it might be almost said that a sleepless watch of the heavens has been kept up by an all-seeing photographic eye, with an accuracy of which the world has hardly had a conception. The completeness with which its work has been done has recently been shown in a striking way. Our readers are doubtless acquainted with the singular character of the minor planet Eros, whose orbit passes through that of Mars, as one link of a chain passes through another, and which comes nearer the earth at certain times than any other celestial body, the moon excepted. When the character of the orbit became established, it was of interest to know whether the planet had ever been observed as a fixed star at former oppositions. Chandler, having computed the path of the planet at the most important of the oppositions, beginning with 1892–94, communicated his results to Director Pickering, and suggested a search of the Harvard photographs to see if the planet could be found on them. The230 result was the discovery of the planet upon more than a score of plates taken at various times during the preceding ten years. New stars were formerly supposed to be of very rare occurrence, but since the Harvard system of photographing the heavens has been introduced, no less than three have been known to break out.
The great revelations of our times have come through the application of the spectroscope to the measurement of motions in the line of sight from us to a star. No achievement of the intellect of man would have seemed farther without the range of possibility to the thinker of half a century ago, than the discoveries of invisible bodies which are now being made with this instrument. The revelations of the telescope take us by surprise. But, if we consider what the thinker alluded to might regard as attainable, they are far surpassed by those of the spectroscope. The dark bodies, planets, we may call them, which are revolving round the stars, must be forever invisible in any telescope that it would be possible to construct. They would remain invisible if the power of the instrument were increased ten thousand times. And yet, if there are inhabitants on these planets, our astronomers could tell them more of the motions of the world on which they live than the human race knew of the motions of the earth before the time of Copernicus.
The men and institutions which have contributed to this result are so few in number that it will not be tedious to mention at least the principal actors. The possibility of measuring the motions of the stars in the line of sight by means of the spectroscope was first pointed out by Mr. now Sir William Huggins. He actually put the method into operation. As soon as its feasibility was demonstrated it was taken up at Greenwich. In these earlier attempts, eye methods alone were used, and the results were not always reliable. Then spectrum photography was applied at the astrophysical observatory at Potsdam by Vogel. Thence the photographic method soon spread to Meudon and Pulkova. But, as often happens when new fields of research are opened, we find them ablaze in quarters where we should least expect. The successful application of the method requires not only the best spectroscope, but the most powerful telescope at command. Ten years ago the most powerful telescope in the world was at the Lick Observatory. Mr. D. O. Mills put at its eye end the best spectrograph that human art could make at that time, the work of Brashear. It is Campbell, who, with this instrument, has inaugurated a series of discoveries in the line in question which are without a parallel.
A mere survey of what has been done in the various lines we have mentioned would be far from giving an idea of the real significance of the advance we are considering. Cataloguing the stars, estimating their magnitudes, recording and comparing their spectra and determining231 their motions, might be considered as, after all, barren of results of the highest human interest. When we know the exact position of every star in the heavens, the direction in which it is moving and the character of its spectral lines, how much wiser are we?
What could hardly have been foreseen fifty years ago, is that these various classes of results are now made to combine and converge upon the greatest problem which the mind of man has ever attempted to grasp—that of the structure of the universe. The study of variable stars has suddenly fallen into line, so to speak, so that now, it is uniting itself to the study of all the other subjects to give us at least a faint conception of what the solution of this problem may be.
One of the principal objects of the present chapter is to make a comparison of these various researches, and discuss the views respecting the constitution of the stars individually, as well as of the universe as a whole, to which they lead us. But there are a number of details to be considered singly before we can combine results in this way. Our early chapters will therefore be devoted to the special features and individual problems of stellar astronomy which have occupied the minds of astronomers from the beginning of their work to the present time. Keeping these details in mind, we can profitably proceed to the consideration of the general conclusions to be drawn from them.
We may begin by refreshing our memories on some points, an understanding of which must be taken for granted. What are familiarly known as the heavenly bodies belong to two classes. Those nearest to us form a sort of colony far removed from all the others, called the solar system. The principal bodies of this system are the sun and eight great planets with their moons, revolving round it. On one of the planets, small when compared with the great bodies of the universe, but large to our every-day conceptions, we dwell. The other planets appear to us as stars. Four of them, Venus, Mars, Jupiter and Saturn, are distinguished from the fixed stars by their superior brightness and characteristic motions. Of the remaining three, Mercury will only rarely excite notice, while Uranus and Neptune are as good as invisible to the naked eye.
The dimensions of the solar system are vast when compared with any terrestrial standard. A cannon shot going incessantly at its utmost speed would be a thousand years in crossing the orbit of Neptune from side to side. But vast as the dimensions are, they sink into insignificance when compared with the distance of the stars. Outside the solar system are spaces which, so far as we know, are absolutely void, save here and there a comet or a meteor, until we look far outside the region which a cannon shot would cross in a million of years.
The nearest star is thousands of times farther away than the most distant planet. Scattered at these inconceivable distances are the bodies232 to which our attention is directed in the present work. If we are asked what they are, we may reply that the stars are suns. But we might equally well say that the sun is one of the stars; a small star, indeed, surrounded by countless others, many of which are much larger and brighter than itself. We shall treat our theme as far as possible by what we may call the natural method, beginning with what, being most obvious to the eye, was first noticed by man, or will be first noticed by an observer, and tracing knowledge up step by step to its present state.
Several features of the universe of stars will be evident at a glance. One of these is the diversity of the apparent brightness, or, in technical language, of the magnitudes of the stars. A few far outshine the great mass of their companions. A greater number are of what we may call medium brightness; there is a yet larger number of fainter ones, and about one half of all those seen by a keen eye under favorable conditions are so near the limit of visibility as to escape ordinary notice. Moreover, those which we see are but an insignificant fraction of the number revealed by the telescope. The more we increase our optical power, the greater the number that come into view. How many millions may exist in the heavens it is scarcely possible even to guess. The photographic maps of the heavens now being made probably show fifty millions, perhaps one hundred millions or more.
Another evident feature is the tendency of the brighter stars to cluster into groups, known as constellations. The latter are extremely irregular, so that it is impossible to decide where one constellation should end and another begin, or to which constellation a certain star may belong. Hence, we can neither define the constellations nor say what is their number, and the division of the stars among them is a somewhat arbitrary proceeding.
A third feature is the Milky Way or Galaxy, which, to ordinary vision, appears as an irregular succession of cloud-like forms spanning the heavens. We now know that these seeming clouds are really congeries of stars too small to be individually visible to the naked eye. We shall hereafter see that the stars of the Galaxy form, so to speak, the base on which the universe appears to be constructed. Each of these three features will be considered in its proper place.
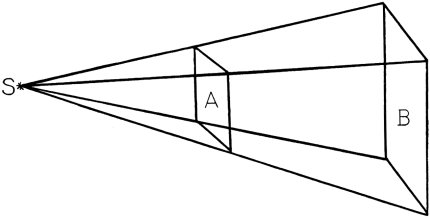
The apparent brightness of a star, as we see it from the earth, depends upon two causes—its intrinsic brilliancy or the quantity of light which it actually emits, and its distance from us. It follows that if all the stars were of equal intrinsic brightness we could determine their relative distances by measuring the respective amounts of light which we receive from them. The quantity of light in such a case varies inversely as the square of the distance. This will be made233 evident by Fig. 1, where S represents the position of a star, regarded as a luminous point, while A and B are screens placed at such a distance that each will receive the same amount of light from the star. If the screen B is twice as far as the screen A, its sides must be twice as large as those of A in order that it shall receive all the light that would fall on A. In this case its surface will be four times the surface of A. It is then evident that any small portion of the surface of B will receive one fourth as much light as an equal portion of surface A. Thus an eye or a telescope in the position B will receive from the star one fourth as much light as in the position A, and the star will seem one fourth as bright.
The fact is, however, that the stars are very unequal in their actual brightness, and in consequence the apparent magnitude of a star gives us no clue to its distance. Among the nearer of the stars are some scarcely, if at all, visible to the naked eye, while among the brighter ones are several whose distances are immeasurably great. A remarkable example is that of Caropes, the second brightest star in the heavens.
For these reasons astronomers are obliged to content themselves, in the first place, with determinations of the actual amount of light that the various stars send to us, or their apparent brilliancy, without regard to their distance or actual brilliancy. The ancient astronomers divided all the stars they could see into six classes, the number expressing the apparent brightness being called the magnitude of the star. The brightest ones, numbering in all about fourteen, were said to be of the first magnitude. The fifty next in brightness were said to be of the second magnitude. Three times as many, an order fainter, were of the third magnitude. The progression was continued up to the sixth magnitude, which included those which were barely visible.
As the stars are actually of every degree of apparent brilliancy, no sharp line of demarkation could be drawn between those of one magnitude and those of the magnitude next higher. Hence, different234 observers made different estimates, some calling a star of the second magnitude which others would call of the first, while others would designate a star of the third magnitude which others would call of the second. It is therefore impossible to state with absolute numerical precision what number of stars should be regarded of one magnitude and what of another.
An idea of the magnitude of a star can be readily gained by the casual observer. Looking at the heavens on almost any cloudless evening, we may assume that the two, three or more brightest stars which we see are of the first magnitude. As examples of those of the second magnitude, may be taken the five brightest stars of the Dipper, the Pole Star and the brighter stars of Cassiopeia. Some or all of these objects can be seen on any clear night of the year in our latitude. Stars of the third magnitude are so numerous that it is difficult to select any one for comparison. The brightest star of the Pleiades is really of this magnitude, but it does not appear so in consequence of the five other stars by which it is surrounded. At a distance of 15° from the Pole Star, Beta Ursa Minoris is always visible, and may be distinguished by being slightly redder than the Pole Star; it lies between two fainter stars, the brighter of which is of the third and the other of the fourth magnitude. The five readily visible but fainter stars of the Pleiades are about of the fourth magnitude. Of the fifth magnitude are the faintest stars which are easily visible to the naked eye, while the sixth comprises those which are barely visible with good eyes.
Modern astronomers, while adhering to the general system which has come down to them from ancient times, have sought to give it greater definiteness. Careful study showed that the actual amount of light corresponding to the different magnitudes varied nearly in geometrical progression from one magnitude to another, a conclusion which accords with the well-known psychological law that the intensity of sensation varies by equal amounts when the exciting cause varies in geometrical progression. It was found that an average star of the fifth magnitude gave between two and three times as much light as an average one of the sixth; one of the fourth gave between two and three times as much light as one of the fifth; and so on to the second. In the case of the first magnitude, the diversity is so great that it is scarcely possible to fix an average ratio. Sirius, for example, is really six times as bright as Altair, which is commonly taken as a standard for a first magnitude star. To give precision to their estimates, modern astronomers are gradually seeking to lay the subject of magnitudes on an exact basis by defining a change of one unit in the magnitude as corresponding to an increase of about two and one half times in the amount of light.
235 If the practice of separating the visible stars into only six orders of magnitude were continued without change, we should still have the anomaly of including in one class stars of markedly different degrees of brightness. Some more than twice as bright as others would be designated of the same magnitude. Hence, to give quantitative exactness to the results, a magnitude is regarded as a quantity which may have any value whatever, and may be expressed by decimals—tenths or even hundredths. Thus, we may have stars of magnitude 5.0, 5.1, 5.2, etc., or we may even subdivide yet farther and speak of stars having magnitudes 5.11, 5.12, etc. Unfortunately, however, there is as yet no way known of determining the amount of light received from a star except by an estimate of its effect upon the eye. Two stars are regarded as equal when they appear to the eye of equal brilliancy. In such a case the judgment is very uncertain. Hence, observers have endeavored to give greater precision to it by the use of photometers,—instruments for measuring quantities of light. But even with this instrument the observer must depend upon an estimated equality of light as judged by the eye. The light from one star is increased or diminished in a known proportion until it appears equal to that of another star, which may be an artificial one produced by the flame of a candle. The proportion of increase or diminution shows the difference of magnitude between the two stars.
As we proceed to place the subject of photometric measures of star light on this precise basis we find the problem to be a complex one. In the first place not all the rays which come from a star are visible to our eyes as light. But all the radiance, visible or invisible, may be absorbed by a dark surface, and will then show its effect by heating that surface. The most perfect measure of the radiance of a star would therefore be the amount of heat which it conveys, because this expresses what is going on in the body better than the amount of visible light can do. But unfortunately the heating effect of the rays from a star is far below what can be measured or even indicated by any known instrument. We are therefore obliged to abandon any thought of determining the total amount of radiation and confine ourselves to that portion which we call light.
Here, when we aim at precision, we find that light, as we understand it, is properly measured only by its effect on the optic nerve, and there is no way of measuring this effect except by estimation. Thus, all the photometer can do is to give us the means of increasing or diminishing the light from one star, so that we can make it equal by estimation to that from some other star or source of light.
The difficulty of reaching strict results in this way is increased by the fact that stars are different in color. Two lights can be estimated as equal with greater precision when they are of the same color than236 when their colors are different. An additional source of uncertainty is brought in by what is known as the Purkinje phenomenon, after the physicist who first observed it. He found that if we took two lights of equal apparent brightness, the one red and the other green, and then increased or diminished them in the same proportion, they would no longer appear equal. In other words, the geometrical axiom that halves or quarters of equal quantities are themselves equal, does not apply to the effect of light on the eye. If we diminish the two equal lights, we find that the green will look brighter than the red. If we increase them in the same proportion, the red will look brighter than the green. In other words, the red light will, to our vision, increase or fade away more rapidly with a given amount of change than the green light will.
It is found in recent times that this law of change does not extend progressively through all spectral colors. It is true that as we pass from the red to the violet end of the spectrum the yellow fades away less rapidly with a given diminution than does the red, and the green still less rapidly than the yellow. But when we pass from the green to the blue, it is said that the latter does not fade out quite so fast as the green.
One obvious conclusion from all this is that two stars of different colors which look equal to the naked eye will not look equal in the telescope. The red or yellow star will look relatively brighter in a telescope; the green or bluish one relatively brighter to the naked eye.
In recent times stars have been photographed on a large scale. Their magnitudes can then be determined by the effect of the light on the photographic plate, the impression of the star, as seen in a microscope, being larger and more intense as the star is brighter. But the magnitude thus determined is not proportional to the apparent brightness as seen by the eye, because the photographic effect of blue light is much greater than that of red light having the same apparent brightness. In fact, the difference is so great that, with the chemicals formerly used, red light was almost without photographic effect. Even now, what we measure in taking the photograph of a star is almost entirely the light in the more refrangible portions of the spectrum. It appears, therefore, that when a blue and a yellow star, equally bright to the naked eye, are photographed, the impression made on the negative by the blue star will be greater than that made by the yellow one. A distinction is therefore recognized between photographic and visual magnitudes.
The photographic magnitudes of the stars are now being investigated and catalogued on a scale even larger than that on which we have studied the visual magnitudes. Yet we have to admit the non-correspondence of the two systems. The bluer the star, the brighter will be its photographic as compared with its visual magnitude. The237 most that can be done is to bring about the best attainable agreement between the two systems in the general average of all the stars.
Fortunately the differences between the colors of the stars are by no means so great as those between the colors of natural objects around us. All the stars radiate light of all colors; and although the difference is quite appreciable either by the eye or by the photograph, it is not so great as it would have been were the variations in color as wide as in the case of terrestrial objects.
Two comprehensive surveys of the heavens, intended to determine as accurately as possible the magnitudes of all the brighter stars, have recently been undertaken. One of these is the Harvard photometry, commenced by Professor Pickering at the Harvard Observatory, and now extended to the Southern Hemisphere by the aid of a branch establishment at Arequipa, Peru.
The instrument designed by Professor Pickering for his purpose is termed a meridian photometer, and is so arranged that the observer can see in the field of his telescope a reflected image of the Pole Star, and, at the same time, the image of some other star while it is passing the meridian. By a polarizing apparatus the image of the star to be measured is made to appear of equal brightness with that of the Pole Star, and the position of a Nicol prism, which brings out this equality, shows the ratio between the magnitudes of the two stars.
The other survey, with the same object, is now being made at the Potsdam Astrophysical Observatory, near Berlin. In the photometer used by the German astronomers the image of one star is compared with an artificial star formed by the flame of a candle. The work is performed in a more elaborate way than at the Harvard Observatory, and in consequence, only that part of the heavens, extending from the equator to 40° north declination, has been completed and published. A comparison of the results thus obtained with those of Professor Pickering, shows a curious difference depending on the color of the star. In the case of the reddest stars, the estimates are found to be in fairly close agreement, Pickering’s being a little the fainter. But in the case of the white or bluish stars, the estimates of the German astronomers are more than one fourth of a magnitude greater than those of Pickering. This corresponds to an increase of nearly one fifth in the brightness. Whether this difference is to be regarded as purely psychological or due to the instruments used, is an interesting question which has not yet been settled. It is difficult to conceive how different instruments should give results so different. On the other hand, the comparisons made by the Germans make it difficult to accept the view that the difference is due purely to the personality of the observers. There are two German observers, Drs. Müller and Kempf, whose results agree with each other exactly. On the other hand, Pritchard, at Oxford,238 made quite an extensive photometric survey, using an instrument by which the light of one star was cut down by a wedge-shaped dark glass, whereby any gradation of light could be produced. A comparison shows that the results of Pritchard agree substantially with those of Pickering. It is quite possible that the Purkinje phenomenon may be the cause of the difference, the source of which is eminently worthy of investigation.
This fact simply emphasizes the lack of mathematical precision in photometric measurements of star light. Even apart from this difference of color, the estimates of two observers will frequently differ by 0.2 and sometimes by even 0.3 of a magnitude. These differences correspond roughly to 20 or 30 per cent in the amount of light.
It must not be supposed from this that such estimates are of no value for scientific purposes. Very important conclusions, based on great numbers of stars, may be drawn even from these uncertain quantities. Yet, it can hardly be doubted that if the light of a star could be measured from time to time to its thousandth part, conclusions of yet greater value and interest might be drawn from the measures.
We have said that in our modern system the aim has been to so designate the magnitudes of the stars that a series of magnitudes in arithmetical progression shall correspond to quantities of light ranging in geometrical progression. We have also said that a change of one unit of magnitude corresponds to a multiplication or division of the light by about 2.5. On any scale of magnitude this factor of multiplication constitutes the light-ratio of the scale. In recent times, after much discussion of the subject and many comparisons of photometric measures with estimates made in the old-fashioned way, there is a general agreement among observers to fix the light ratio at the number whose logarithm is 0.4. This is such that an increase of five units in the number expressing the magnitude corresponds to a division of the light by 100. If, for example, we take a standard star of magnitude one and another of magnitude six, the first would be 100 times as bright as the second. This corresponds to a light ratio slightly greater than 2.5.
When this scale is adopted, the series of magnitudes may extend indefinitely in both directions so that to every apparent brightness there will be a certain magnitude. For example, if we assign the magnitude 1.0 to a certain star, taken as a standard, which would formerly have been called a star of the first magnitude, then a star a little more than 2.5 times as bright would be of magnitude one less in number, that is, of magnitude 0. The one next brighter in the series would be of magnitude -1. So great is the diversity in the brightness of the stars formerly called of the first magnitude that Sirius is still brighter than239 the imaginary star just mentioned, the number expressing its magnitude being -1.4.
This suggests what we may regard as one of the capital questions in celestial photometry. There being no limit to the extent of the scale, what would be the stellar magnitude of the sun as we see it when expressed this way on the photometric scale? Such a number is readily derivable when we know the ratio between the light of the sun and that of a star of known magnitude. Many attempts have been made by observers to obtain this ratio; but the problem is one of great difficulty, and the results have been extremely discordant. Amongst them there are three which seem less liable to error than others; those of Wollaston, Bond and Zöllner. Their results for the stellar magnitude of the sun are as follow:
| Wollaston | -26.6 |
| Bond | -25.8 |
| Zöllner | -26.6 |
Of these, Zöllner’s seems to be the best, and may, therefore, in taking the mean, be entitled to double weight. The result will then be:
| Stellar magnitude of sun | -26.4 |
From this number may be readily computed the ratio of sunlight to that of a star of any given magnitude. We thus find:
The sun gives us:
| 10,000,000,000, | the light of Sirius. |
| 91,000,000,000, | the light of a star of magnitude 1. |
| 9,100,000,000,000, | the light of one of magnitude 6. |
The square roots of these numbers show the number of times we should increase the actual distance of the sun in order that it might shine as a star of the corresponding magnitude. These numbers and the corresponding parallax are as follows:
| Sirius; | Distance = | 100,000: | Parallax = | 2″.06 | |
| Mag. | 1 | ” | 302,000: | ” | 0″.68 |
| ” | 2 | ” | 479,000: | ” | 0″.43 |
| ” | 3 | ” | 759,000: | ” | 0″.27 |
| ” | 4 | ” | 1,202,000: | ” | 0″.17 |
| ” | 5 | ” | 1,906,000: | ” | 0″.11 |
| ” | 6 | ” | 3,020,000: | ” | 0″.07 |
These parallaxes are those that the sun would have if placed at such a distance as to shine with the brightness indicated in the first column. They are generally larger than those of stars of the corresponding magnitudes, from which we conclude that the sun is smaller than the brighter of the stars.
In a previous paper I reviewed briefly the history of preventive inoculation and described the results of my attempts to secure a ‘virus fixé’ in the case of cholera. It will be remembered that the two vaccines finally obtained protected guinea pigs successfully against all possible forms of cholera infection.
It was now necessary to ascertain whether the same protection could be given to man which was observed in animals. For this purpose it was essential to first of all prove the perfect harmlessness of the operation. This was established by very careful observations of medical men and scientists who were inoculated in Europe soon after the results of the above investigations were published. The inoculation causes a rise of temperature and general discomfort, which lasts one or two days, and some pain at the seat of the injection, which disappears in a few days. The fever and discomfort induced are, on the whole, shorter in duration, though often more intense, than those caused by vaccination against smallpox. The effect disappears within a few days and the individual returns to his usual condition of health.
The next and all-important stage was to devise an experiment or a series of experiments on man so as to test the efficiency of the method against cholera attacks. This part of the investigation could only be done in a cholera-stricken country, where opportunities would arise of comparing the incidence of the disease in inoculated and uninoculated. Such opportunities are limited. Except in certain parts of India and China, cholera appears in localities unexpectedly and does not last long. In the places where the disease is endemic the cases are scattered over large areas. These features rendered the demonstration of the effect of the vaccine a matter of particular difficulty. In 1893 I went to India, and in the course of a year inoculated some twenty-three thousand people in the northern parts of the country; but no cholera appeared in their midst to show whether the vaccine was of value or not. In the spring of 1894 the inoculations were introduced into Bengal, and, with the assistance and co-operation of Prof. W. J. Simpson, of King’s College, London, at that time Health Officer of Calcutta, and of his staff, efforts were made to induce the inhabitants of the bustees of Calcutta to get themselves inoculated. These bustees are isolated villages consisting of groups of mud huts inhabited by the poorer class.241 Owing to the consumption of water from the ponds or tanks belonging to these villages, the inhabitants of the bustees are subject to periodic visitations of cholera. It was in one of these bustees that the first observation was made as to the effect of the cholera vaccines.
The spring is essentially the cholera season in Calcutta. About the end of March two fatal cases of cholera and two cases of choleraic diarrhœa occurred in Katal Bagan Bustee, in a population grouped around two tanks. This outbreak led to the inoculation of one hundred and sixteen persons in the bustee out of about two hundred. After the inoculation there occurred nine more cases of cholera, seven of which proved fatal, and one case of choleraic diarrhœa. All the ten cases occurred among the uninoculated portion of the inhabitants, which formed the minority, none of the inoculated suffering. The results were more interesting when analyzed in detail. Some of the cases had occurred in families in which some of the members had been inoculated and others not, and the disease selected the non-inoculated members, sparing the inoculated. Thus, in one house six members out of eight had been inoculated. The attack, a fatal one, occurred in one of the remaining two. In another house eleven members out of eighteen were inoculated. The eleven members remained free while four out of seven not inoculated were attacked.
Upon these observations the Calcutta municipality felt encouraged to vote funds for the continuance of the inoculations in an experimental farm, and appointed for that purpose a special staff. In 1896 the result of two years’ observations were embodied by the health officer in a report to the Calcutta Municipal Corporation. It recorded a most satisfactory state of affairs. During the time under observation some eight thousand persons were inoculated. Cases of cholera occurred in seventy-seven huts in which some members of the family had been previously inoculated and others not. Comparing the incidence of the disease in the two groups, a striking advantage was found to be with the inoculated. I made an analysis of the cases according to the time which had elapsed between inoculation in each of these huts and the occurrence of cholera in them, and the following results were found. During the first four days after inoculation, apparently before the vaccine had time to produce its full protective effect, there were proportionately 1.86 times fewer deaths among the inoculated than among the non-inoculated members of the families. In a second period, extending from the fifth to the four hundred and twenty-ninth day—i. e., for fourteen months—there were 22.62 times fewer deaths among the inoculated; while in the last period—that is, between the four hundred and thirtieth and seven hundred and twenty-eighth day after the inoculation—there were only 1.54 fewer deaths among the inoculated, the immunity having evidently gradually disappeared. The net242 result was that for two years after inoculation, including the periods of incomplete protection, there was a reduction in mortality of 72.47 per cent among the inoculated; or in other words, in houses in which inoculations were performed and in which cholera subsequently occurred there were, even from the day of inoculation, before the full effect of it could be produced, eleven deaths among the non-inoculated to only three among the inoculated. Eight lives out of every eleven were saved.
At the end of my first cholera campaign, in August, 1895, there were altogether 31,056 natives of India, 125 Eurasians, 869 Europeans of the civil population, 6,627 native officers and sepoys, and 294 officers with 3,206 men of the British troops stationed in India, in all 41,787 people, who had submitted to inoculation. Observations instituted among them, especially among prisoners, soldiers and coolies in tea estates, with regard to whom detailed records could be kept, went to confirm the results as detailed above. In order to lengthen, if possible, the period of immunity, the plan was formed of inoculating stronger vaccines and in higher doses. The inoculations are now carried on in a Government laboratory, in Purulia, Bengal, chiefly among the people emigrating to the cholera districts of Assam, and there is no doubt that in the course of time a marked effect upon the prevalence of cholera in those districts will be produced and valuable theoretical data will be obtained.
There was one noticeable feature about the results of the inoculation against cholera which early attracted my attention, and this was that while the number of attacks and the absolute number of deaths was strikingly influenced by the operation, the proportion of deaths to those attacked did not appear to be changed. The case incidence was effectively checked, but the ‘case mortality’ was not reduced. The inoculation diminished the chances of an attack of cholera—that is, the chances of the cholera virus penetrating into the tissues of a man; but if it so happened that the patient was attacked and the virus found an entrance and started growing in the system notwithstanding the inoculation, the latter would not assist in mitigating the severity of the symptoms or reducing the fatality of the disease. In analyzing this result further, it seemed to me permissible to assume that the vaccine protected against the cholera microbes themselves, but did not protect against their poisonous products, which are the cause of the actual symptoms.
This interpretation of the facts found support in a set of laboratory experiments by Professor Pfeiffer and Dr. Kolle, of Koch’s Institute, in Berlin, who showed that the blood serum of animals and persons inoculated with the cholera vaccine, as practiced in India, acquired an243 intense power of destroying cholera microbes, but exhibited no properties capable of counteracting the effect of their toxic products—no ‘antitoxic properties’. Combined with those of previous experimenters these results tended to prove that two kinds of immunity could be produced separately, and it became incumbent to devise a plan which would secure not only a lowering of susceptibility to the disease, but also a reduction in the case mortality.
For that purpose it seemed rational to attempt the treatment with a vaccine containing a combination of bodies of microbes, together with their toxic products. I intended to test this plan experimentally in the cholera districts; but, plague having broken out in Bombay, the Government of India commissioned me to inquire into the bacteriology of that disease, and I determined that the knowledge gained in the cholera inoculations should be applied and tested in the preparation of a prophylactic against the new epidemic.
The experiments I had in view involved manufacturing a material on a large scale, and operating on it for weeks continuously. To do this it was essential to find a way of recognizing plague growth with certainty, so as to enable the officers engaged in the manufacture to control the process and know exactly when they were handling the proper stuff, and when an admixture and invasion of extraneous growth took place. When this was solved, a drug was prepared by cultivating the plague microbe in sterilized broth, to which a small quantity of clarified butter or of cocoanut oil had been added. The plague bacilli attach themselves to the drops of butter or oil floating on the surface, and grow down into the depth of the liquid, forming a peculiar threadlike appearance. While doing so they secrete toxic matter, which is gradually accumulated in the liquid; at the same time a large amount of microbial growth comes gradually down from the surface of the liquid and collects at the bottom of the flask. When shaken up the whole represents the desired combination of the bodies of microbes and of their toxic products. The process is continued for a period of five to six weeks. As the microbes of plague had been very little studied before, and as their exact effect on the human system was unknown, I decided not to use for the treatment living microbes, but to use at least at first ‘carbolized’ vaccines, though the result of the treatment might be less favorable or less lasting than that which could be expected from living vaccines. The microbes in the above plague growth were accordingly killed by heating them at a temperature ranging from 65° to 70° C., and then mixed with a small proportion of carbolic acid, to prevent the drug from subsequent contamination and decomposition. The dose of the prophylactic was regulated by measuring up the quantity to be injected. The requisite amount is determined by the degree of fever which it produces. The febrile244 reaction varies in different individuals, but a temperature reaching 102° and above in at least thirty per cent of those inoculated has been found to indicate a good material. In the cholera, rabies and smallpox vaccines, the microbes being employed in a living state, it was essential to fix the strength of the vaccine, for otherwise it was impossible to predict the behavior of the microbe when injected into the system. In the case of the plague prophylactic the activity of the microbes is arrested before it is inoculated, and the effect can be regulated, as mentioned above, by simply measuring up the doses in the same way as is done with any chemical drug.
The expectation formed when devising the plan for the plague prophylactic has been very fortunately justified, and an advance on the results from the cholera vaccines was obtained; but I can not yet say certainly whether this favorable result is indeed due to the particular provisions which I had made for obtaining it.
The effect of the plague prophylactic was first tested at the Byculla Jail, in Bombay, when the epidemic reached that establishment. From the first day after the inoculation till the end of the outbreak there were in the jail twelve cases and six deaths among one hundred and seventy-two uninoculated inmates, and two cases, with no deaths, among one hundred and forty-seven inoculated. A year later, almost exactly a similar result was observed when the plague attacked the so-called Umarkhadi Common Jail, in Bombay. In this case after the inoculation there were ten cases and six deaths among one hundred and twenty-seven uninoculated inmates, and three cases, with no deaths, among one hundred and forty-seven inoculated. These and other observations show that the vaccine for the plague begins to exercise its effect within some twenty-four hours after inoculation; that it is useful even in the case of persons already infected; that it is therefore applicable at any stage of an epidemic. Numerous further observations were soon collected on the working of the system.
At the small village of Uudhera, of the Baroda feudatory state, where plague broke out, inoculation was applied to a half of each family, the other half remaining uninoculated. After that there were twenty-seven cases and twenty-six deaths among sixty-four uninoculated, and eight cases, with three deaths, among seventy-one inoculated of the same households, the proportionate difference in mortality being over eighty-nine per cent. There followed observations on a far larger scale, demonstrating that the mortality of the inoculated, compared to that of the non-inoculated, was on an average between eighty and ninety per cent less. Sometimes this reduction reached ninety per cent. In the Punjaub, in a village called Bunga, there occurred, in two hundred and eighty-one not inoculated, ninety-seven cases of plague and sixty-five deaths, while among seventy-four inoculated there245 were six cases, but no deaths. In Bangalore, among 80,285 of the inhabitants not inoculated, there were 2,208 deaths from plague, while among 23,537 inoculated there were only 108. The observations at Lanowli, Kirkee, Daman, Hubli, Dharwar, Gadag, in the Bombay Presidency, gave the same results. At Hubli over forty-two thousand inhabitants out of some fifty thousand were inoculated. In Bombay city, out of a population of 821,764, 157,256 have now undergone the inoculation. The work proceeds here at present at the rate of one thousand to eleven hundred inoculations a day.
From plague hospitals the returns show that among those of the attacked who were previously inoculated the mortality is reduced to less than one half of that among patients who were not inoculated. The property of reducing the case mortality thus appears to belong to the plague prophylactic in an unmistakable degree.
By the anti-cholera and anti-plague inoculation the methods of preventive treatment by means of cultivated bacteria and their products have been rendered, so to say, a part of the daily policy in human medicine. The usefulness and practicability of those methods have become clearly apparent, and steps have been taken to extend further the field of their application. On the ground of the experiments made with the typhoid bacillus in the Pasteur Institute in 1889–’93, and of the results obtained from the anti-cholera inoculation in India, I was able to induce Professor Wright, of the Pathological Laboratory in Netley, whom I initiated in 1892 in the principles and technique of anti-cholera inoculation, to start a campaign of similar operations against typhoid among the British troops. The latter are stationed at different times of their service very nearly in all parts of the world, and yearly pay a very heavy tribute to that disease. The medical officers in charge of these troops pass through a course of training at Netley, and Professor Wright had rendered excellent services in connection with the cholera inoculations, by disseminating the knowledge of them among the probationers of the school. It seemed to me expedient, therefore, to start the typhoid inoculation also through the staff and pupils of that school. The following plan as to the preparation of the vaccine, and the way of carrying out the inoculation, was laid before Professor Wright. The typhoid bacillus was to be brought to a fixed stage of virulence by the inoculation in the peritoneal cavity of Guinea pigs, according to the exact rules prescribed for the anti-cholera inoculation. Once the virus was fixed, it was to be cultivated for twenty-four hours on a solid medium, and a first vaccine prepared by carbolizing that virus. As, however, the durability of the effect of carbolized vaccine alone was not known, this was to be followed up by the injection of a dose of the fixed living virus.
246 The inoculation was first to be made on volunteers among the physicians on probation at Netley; then on volunteers among the young officers of the army on the eve of their departure for the tropics; and then, with the approval of the military authorities, on volunteers among private soldiers. At the end of 1895, during my visit to England, I obtained from Sir William Mackinnon, then Director-General of the Army Medical Department, permission for Professor Wright to start the work upon the plan above detailed; and the first inoculations, in the way described above, were done in the middle of 1896. Soon after that, Pfeiffer and Kolle, recognizing the same similarity between the cholera and typhoid microbes, and pointing out that the results obtained by us in India were likely to be repeated when applying the method to typhoid, proposed and started a similar series of inoculations.
When the inoculation against plague was begun, and observation showed that dead vaccines alone were apparently sufficient to produce satisfactory results, a second inoculation with living virus appeared less urgently necessary; and as the effect of such an inoculation, which Professor Wright very courageously tried first on himself, seemed troublesome, it was decided to do for the time being the second inoculation also with the carbolized virus. Similarly, the plan which was adopted for the plague inoculation, of cultivating the vaccine in a liquid, instead of a solid medium, and of using cultures of several weeks’ duration, has been subsequently adopted in the typhoid inoculation also.
Many thousands of British soldiers and civilians have already undergone the inoculation in question. The latter was done partly with vaccines cultivated on a solid medium, according to the older plan, and partly with vaccines prepared according to the plague inoculation method. The results so far observed are encouraging, and, I hope, will shortly be improved considerably. At the last Harveian dinner in London, Surgeon-General Jameson, Director-General of the Army Medical Department, summarized the results of the observations in India, where, among several thousands of young soldiers, the most prone to the disease, the incidence of typhoid since their inoculation was 0.7 per mille, while among the older, more resistant, not inoculated soldiers, the incidence was during the same period just double that. A large proportion of the force now on service in the South African campaign have been inoculated, some before embarking and others on their way out.
Such is the position of preventive inoculation, as applied, so far, to human communities. The very success of these operations is now apt to create some sort of feigned or earnest alarm, and one meets at present with the question, What is going to happen to our poor247 body if we are to be inoculated against all diseases? and with this other one, How do you expect us to make a living if you try to keep all of us alive? The humorous form of these questions usually permits of their dropping out of the conversation without a reply. The earnest answers are, however, obvious. The efforts of the bacteriologists in combating diseases are at present directed to a twofold aim: their prevention, by a prophylactic treatment, and their cure. The advantage of a curative treatment is that it is to be applied to a relatively small number of persons, to those who actually fall victims to an attack; while that of the preventive treatment is in the greater certainty with which safety and protection are secured by it. The relative position of the two treatments will, in practice, differ in different diseases—namely, according to the prevalence and fatality of a given disease, and according to the merits of the two treatments as they stand at the time. In diseases in which the risks of being attacked are smaller, or the consequences of an attack less serious, or for which a very effective and sure curative treatment has been discovered, the majority of people will prefer to wait for an actual attack rather than to undergo the discomfort of a preventive treatment; in diseases, on the contrary, in which the chances of being attacked are great, or in which the fatality is higher, the sequelæ of an attack more serious, and for which a successful and not very troublesome preventive treatment has been found, large numbers will undergo preventive inoculation. But, even in the latter case, a mutual co-operation between the two methods will exist always, as there will always be a number of people, either among those who have neglected to protect themselves by inoculation, or among those in whom the inoculation has proved unsuccessful, who will fall victims to an attack and require the benefits of a curative treatment, be those at the time little or great.
The answer to the second question is of course to be expected rather from the politico-economist, the wise administrator, the civilian, than from the bacteriologist. In any case it is clear already that if we are ever to be told that we must thin our ranks, we shall prefer not to leave the task in the hands of the indiscriminating microbe, but to have some voice in the matter ourselves. Inoculation marks only the conquest of another force which henceforth we shall be glad to control.
Bombay, India, March, 1900.
The growth of the relations between a colony and the mother country closely follows the development of the relationship between an organism and its offspring, or (in higher species) between parents and children. When an infusorian subdivides into two cells, the new cell produced swims away and henceforth leads an independent life. Most of the Phœnician and most of the earlier Greek colonies were social infusoria which parted from the parent organism by segmentation and had no further relations with it. As we rise in the animal scale a new relationship, that between mother and young, and a new instinct, the maternal, come into existence. These begin as low down as the mollusks, and expand and heighten, though not without strange lapses, in both insects and birds as species develope; but we need not trace the evolution here. Let it suffice to note that there are successive degrees of specialization; a site is chosen suitable for depositing and hatching eggs; means are found for making them secure; a shelter is built for them; they are deposited near substances adapted to nourish the young; special food is prepared for them; they are reared through food disgorged or brought to them. The accession of the male to the family marks the dawn of the paternal instinct; it appears earliest among fishes. This evolution is repeated in the history of colonies, where, however, the maternal and paternal offices melt into one another insensibly.
The mother country founds and nurtures colonies. Most of the earliest colonies are the work of adventurous bands or navigating merchants or fishermen, who seek their own habitats, carry with them their own equipment and fight their own battles. Then the metropolis settles its surplus or discontented citizens in territories previously chosen, provides them with all that is necessary for their start, and often nourishes them during the infancy of the colony. Hispaniola was a state colony manned with miners and artisans who were provided with tools, and this at the cost of a loan and a draught from the confiscated property of the Jews. Nor was it until gold began to be found in large quantities that the receipts equalled the expenditure on the young colony. Louisiana was founded and fostered with a royal munificence that conferred on it “more than was contributed by all the English monarchs together for the twelve English colonies on the Atlantic.” Georgia was a one-man249 foundation, but the British Parliament twice granted considerable sums to initiate it and carry it on; the Society for the Propagation of the Gospel aided, and the benevolence of philanthropic England contributed largely to its success. Not till 1818—more than half a century after the conquest—did the revenue of Canada balance its expenditure. The convict colony of New South Wales was, of course, entirely of state origin. Stores of every kind, together with cattle and seeds, were sent out at the beginning, and long continued to be sent out to it. The first governor was granted a space of two years to make it self-supporting, but the growth of a convict colony is abnormally slow, and the civil and military establishments for thirty-four years continued to be a drain on the British exchequer to the extent of over ten millions. Even now one of the oldest and best of existing British colonies, with an area of over three hundred thousand square miles, does not produce the breadstuffs needed for its own consumption. The Cape of Good Hope, of mixed Dutch and French origin, was first made a truly British colony by the dispatch of six thousand emigrants at the cost of the mother country—a cost much greater than was anticipated. When the Transvaal was forcibly annexed by England, the stepmother country advanced a sum of £90,000 to rescue the quondam republic from its financial difficulties. In 1895 Parliament voted three millions for the building of a railroad in British East Africa. Uganda is supported by a British subsidy. Algeria is a manufactured colony, which has all along had to be supported by its creator. Apart from the cost of their civil and military establishments, France has to subsidize her colonies to the extent of over four millions sterling, partially expended in reproductive public works. Even tiny New Caledonia costs France half a million, one half of which, it is true, is expended on the convict establishment.
Most colonies at their beginning are burdensome to the mother country. Years after its foundation South Australia fell into such embarrassment that its governors had to draw on the imperial exchequer for nearly a million. In 1834 the expenditure in Cape Colony was still in excess of the revenue. Sierra Leone had to be aided by a parliamentary grant year after year. No wonder the Colonial Office complained that colonies were expensive to keep up. In German Africa the revenue does not meet the expenditure. The Congo Free State does not pay its way. On the other hand, Congo Française has a substantial surplus. Western Australia was another exception to the rule. There the Imperial Government announced that it would contribute nothing to the foundation of the colony, which was to be self-supporting from the first. Private capitalists were to arrange for the emigration of ten thousand persons in four years. Lands were granted to the emigrants on a scale of extravagance which long hampered the250 progress of the colony. Companies likewise expend large sums in many colonies. French and English companies embarked on American, Indian, African and island adventures at ruinous loss. Law’s company withdrew from Louisiana, the New Zealand Company from New Zealand, and the Canterbury Association from Canterbury with a balance on the wrong side of the account. Wealthy individuals bear their part. Mr. Rhodes annually subsidizes the British Central African Protectorate, and King Leopold the Congo Free State. Colonial bishoprics have also been endowed and colonial cathedrals built, largely with the aid of voluntary contributions by sympathizers in the mother country.
The mother state sometimes gives the colonies the benefit of her financial good name. In 1869 England withdrew her regiments from New Zealand when the colony was still at war with the Maoris, and to salve the wounded feelings of the colonists she agreed (under pressure) to guarantee a loan of a million in aid of emigration and public works. Before the Canadian Pacific Railway could be completed the Imperial Government had to guarantee a loan of £3,600,000. Mr. Rhodes proposes (unsuccessfully, it now appears) that the Imperial Government, which contributed £200,000 to the cost of a railway from Kimberley to Buluwayo, should guarantee a loan of an enormous amount for the continuation of the African trunk railway from Buluwayo to Lake Tanganyika.
The mother country supports or aids its self-governing colonies through its capitalists. In order to execute public works—roads, bridges and railways—to assist immigration, to build fortresses, and sometimes to pay the interest on previous loans, all the colonies have habitual recourse to the British Stock Exchange. There are good reasons for this. The colonies have little capital of their own, for all their money has been used up from day to day. The English investor has an almost unlimited amount—the savings mainly of one industrious century—and he is prepared to lend it at a lower rate of interest than would content the colonial capitalist. Of over two thousand millions sterling which John Bull has out at usury all over the world, the total public and private indebtedness of the seven Australasian colonies alone, with a population of four millions, is stated to exceed three hundred and twenty millions, or at the rate of eighty pounds per head of these daring colonists. One half of this sum is due from colonial governments for the purposes already named. The half of it, due from banks, building companies, mercantile associations and mortgage agencies, excites no misgivings; these institutions can always go bankrupt, as many of them did in the financial collapse of 1891–’93. But it is not open to a British colony to file its schedules, or at least so we used to think; and so the Times said till the oldest of British colonies went bankrupt the other day. At all events, it is harder, and we contemplate251 this enormous pile of public indebtedness in young and scantily peopled communities with the same feelings as made alarmists foresee impending ruin in the growing augmentation of the gigantic public debt of the United Kingdom. It is commonly said that while the imperial debt has been accumulated as the cost of “just and necessary wars,” or of wars that were neither just nor necessary, the colonial debt has been contracted for the execution of reproductive public works. This is not altogether so. Eleven million pounds of the public debt of New Zealand were contracted to carry on war with the Maoris, who were defending their territory. The Seven Years’ War, which was begun on the part of England to gain possession of the Ohio Valley and thus increase the extent of her colonies, doubled her public debt. Where is the difference between the two classes of expenditure? Then most of the self-governing colonies have expended large sums in fortifying ports, some in partly supporting a fleet, and one at least in purchasing war ships of its own. Nor has all the remainder been reproductively expended. The building of schools is a wise way of spending money, one’s own or another’s, but it can not be called a materially reproductive way. Governors’ and ministerial residences, parliamentary and departmental buildings, are indispensable, but they can not be called ‘assets,’ especially if built of perishable and inflammable timber. Even railways, most profitable of public works, are not always true assets. In many of the colonies they are light railways, and when traffic increases and a higher speed is required they will have to be built over again and new rolling stock procured. Not a few of them, too, are ‘political railways,’ running through a sparsely populated country no-whither, and built to capture votes. Roads are only less valuable, but they were made (sometimes by graduates and men of scientific antecedents who were afterward cabinet ministers) at the wage rate of from two guineas to four pounds ten per week, and are an inadequate return on the outlay. Last century British loans were issued as prizes to friends of ministers, and a much reduced amount found its way to the treasury. Deduct an analogous, though not quite similar, item of waste in colonial loans, add this to all the other non-reproductive elements, and the genuinely reproductive proportion will shrink considerably. Every one of the colonies, even with the fee simple of territories only less than Europe in extent in their hands, would have sunk under the increasing burden. Happily or not, the ever-growing wealth of England has so cheapened money that the interest charge on the whole Australasian indebtedness sank in five years (1890–’96), mainly through conversion of loans, from fourteen millions to twelve and a quarter. It may be added that the colonies which have borrowed most recklessly have not been the most populous or those with largest resources, but rather the socialistic colonies with big schemes on hand.
252 A father may assist his son by supplying him with the capital needed to carry on his business. Thus it is entirely with the mother country’s money that the first colonial banks are founded. As the colony grows wealthier and the business of the banks extends, colonial shareholders purchase stock in it, but the number of British shareholders remains considerable. A typical example is that of the Bank of New Zealand, from two fifths to one half of whose shares are (or in 1888 were) held in the United Kingdom. In the older or wealthier colonies of New South Wales and Victoria the number of English shareholders may be smaller, though still large. A still larger proportion of the shares of the great colonial steamship companies, amounting possibly to three fourths or nine tenths of the whole, is held (chiefly by commercial men and firms) in Great Britain. Many commercial undertakings in all the colonies are engineered entirely by English capital (not included in the two thousand millions). The Canadian transcontinental railway; railways, electric tramway lines and silver mines in Tasmania; the Midland Railway and also copper mines in New Zealand; the gold mines in Western Australia to such an extent that much more English capital is said to pour into that colony than gold flows out of it—are only a few colonial enterprises that would never have been undertaken but for the mother country’s aid. Some of these are lucrative, others not; some have been abandoned, and others belong to a still darker class. “Uncounted millions of capital have been raised in the central money market of London, only to be fooled away in ill-conceived and misdirected enterprises abroad,” says Lord Brassey. Nor are the losses confined to questionable undertakings. Two great Australasian banks have frittered away their entire capital of four and three millions, respectively, and it may be assumed that the British investor has borne one half of the losses. Of half a dozen smaller colonial banks a similar tale might be told. Father and son have to share in one another’s adversity, as in one another’s prosperity.
The socialistic movement in England has lately so strongly reacted on the relations of the Imperial Government with the colonies that the Secretary of State is believed to be willing to employ the resources of the empire to assist backward colonies. He has invited English capitalists to aid the declining West Indies, and a leading firm has offered to invest a million in the sugar industry if a guarantee of sufficient returns is given. The constitution of the projected Australian Federation contains a novel analogous provision, permitting the commonwealth to aid its needy provinces. The growing unity in the social organism as a whole is accompanied by an increasing unity in its component parts.
The mother country continues to defend its colonies, as animals defend their young and parents their children. But the polyp does not defend its offspring, nor did the earliest colonizing powers succor253 their colonies. While not even the armed persuasion of Cambyses could induce Tyre to make war against Carthage, neither seems to have helped the other in its need. Carthage fought savagely for her Sicilian colonies, but in her own interests, not in theirs. Though the ties between a Greek metropolis and her colonies were closer, the one did not invariably defend the other. Corcyra refused the aid her daughter city Epidaurus sought, and the latter had to find it in the grandmother city of Corinth, who considered it her colony no less than that of Corcyra. The Dorian city was celebrated for her typical Greek patriotism, and she gladly assisted Syracuse to expel her Carthaginian conquerors. Rome fought for her colonies while her power lasted. France and England fought for their colonies, or rather for the possession of them, all through the eighteenth century. Spain has just fought for her last colonies, but as much against the colonists as against the foreign state that came to set them free. The mother country is also at the cost of keeping her colonies in a state of defence. The sum of £9,000 was in 1679 annually expended on the maintenance of English soldiers in Virginia and two West Indian colonies, and £1,000 on the fortifications of New York. Troops were often dispatched to assist the American colonies in special expeditions. The colonial military expenditure of Great Britain in 1859 amounted to nearly £1,200,000. In compliance with the findings of a Royal Commission, repeatedly reaffirmed by resolutions of Parliament, to the effect that the self-governing colonies ought to suffice for their own military defense, the troops were finally withdrawn in 1873, but she still maintains a garrison at Halifax and in Natal and a fleet in Australian waters, to which last the adjacent colonies contribute a fraction. Most of the self-governing colonies have at their own cost erected fortresses, and they maintain a defensive force. Two of them have stationary ships of war. They are willing and eager, moreover, to aid the mother country when she is in difficulties. When England was embroiled in Egypt or danger threatened in India and South Africa, several of these colonies offered to send, and one actually sent, troops to engage in wars in which they were not directly concerned. The head and the extremities are sometimes at variance because their interests conflict. The heart of such an empire is one. A stride has been taken toward organic unity.
Animals evolve special organs for the nursing of their young, and all colonizing countries seem to have created special departments for the supervision of their colonies. As the lacteal glands are only modified skin-glands, are in certain lower genera (the Monotremata) at first without teats and only in higher species develop into true mammæ, so the colonial department in the mother country is originally a mere adaptation of existing agencies. A rather perfect example of this stage is presented by the earliest of modern colonizing254 powers. The Casa de la Contratacion de las Indias, established soon after the discovery of South America, was organized in 1503. It granted licenses, equipped and despatched fleets, received merchandise for export and cargoes imported and contracted for their sale. It controlled the trade with Barbary and the Canaries and supervised the shipping business of Cadiz and Seville. Taking cognizance of all questions concerning marine trade, it was advised by two jurists. It also kept the Spanish government informed of all that concerned the colonies. It was a general board of colonial marine trade, and such it remained even when, a few years later, its more important colonial functions were absorbed by a higher department.
Where the colony has been founded by a commercial or by a colonizing company, the mother country controls the colony through the directors of the company; the office of the company is pro tanto the Colonial Office. Yet the later colonial department, as an organ of government, is not a development of these shipping, commercial or colonizing boards. It is a delegation of the sovereign authority. This is at first exercised directly by the sovereign as it was notably by Isabella and Ferdinand. It is next delegated, like almost all functions of the ruler, to his privy council, which assigns the business of colonies to a committee, which again may be set apart as an independent administrative body. The Spanish Council of the Indies, the separate English privy council for colonial affairs contemplated in the first Virginian charter, the Council of Nine appointed by the States-General of the Netherlands, the Swedish royal council, were such bodies. Their powers are everywhere the same. The superintendence of the whole colonial system is entrusted to them. They have supreme jurisdiction over all the colonies. They appoint and may recall viceroys, governors-general, governors and other local officers. They can veto laws and ordinances made by colonial rulers or legislatures. They frame constitutions for the colonies and enact laws. Through the governors and other officers sent out by them, they minutely supervise and incessantly interfere with the whole internal administration of each colony. The tendency of this supreme council is to divorce itself evermore from the privy council and become independent, till at last it is transformed into a ministerial department. Yet an amicable relationship (such as sometimes survives the divorce court) long remains. The Colonial Committee of the privy council in England was summoned as late as 1849, and the Judicial Committee still hears appeals from colonial courts of justice. The government of the commonwealth was naturally averse to the king’s council, and a body of special commissioners (Cromwell and Pym and Vane among them) was appointed to govern the colonies.
The Restoration did not at once return to the old system. On the255 contrary, a remarkable democratic advance was made. Recognizing that though ‘politics lie outside the profession of merchants’ (as the Swedish and British governments declared), yet trade is eminently within their scope, the restored monarchy set up a Council of Trade and Plantations, of whose forty members twenty were elected representatives of the five merchant companies and the incorporated trades. But there was ever a tendency, at least under the despotic rule of the Stuarts, to revert to the privy council, and in 1674 a standing committee of it was appointed Lords of the Committee of Trade and Plantations. The change appears to have been unimportant. Trade still governed the committee and shaped its policy.
The Board of Trade set up in 1696, rather by the House of Commons than by the Ministry, marked the more popular character of the revolution of 1688, and lasted for ninety years. As if foreshadowing the despotic character of the English reaction against the greater French revolution, this board was abolished by an act introduced by the chief reactionary—Edmund Burke. A committee of the Privy Council for Trade and Plantations was in 1786 again resorted to, and this committee in a shadowy manner survived (perhaps it still survives) till 1849, when it was for the last time summoned by Earl Grey. But the real administration of the colonies had long been in the hands of a department of state, directly responsible to Parliament, though it was still a department that dealt with other affairs as well. Specialization began in 1702 by the colonies being assigned to the Secretary for the Southern Department. In 1768 a separate department with a secretary was created for America, where almost all of the colonies were then situated. After the loss of most of the American colonies the new department was abolished in 1782. The colonies were then annexed to the home department. In 1794 the newly created war department nominally included the colonies, though these were not actually united with it till the Committee for Trade and Plantations ceased to act, seven years later. In 1854 a separate colonial department, with an independent secretary of state, was finally created.A
A The history of the relations between the government of Great Britain and her colonies will be found in many books, but best in Mr. Egerton’s comprehensive survey of British colonial policy.
As there were twenty-three secretaries in forty-one years, it will be readily understood that the practical work of administration remained with the permanent officials. With a longer tenure of office, previous training and thorough mastery of details, they held all the threads of colonial administration in their own hands. A newly-appointed minister, with little knowledge of the colonies and no acquaintance at all with the business of his department, was no match for an experienced officer who had colonial affairs at his fingers’ ends.
256 A mere clerk, unknown outside his office, though well known in literature, could recall a governor; another, whose very name was unknown till he died, recommended (that is, commanded on pain of dismissal) a recent Governor of New Zealand to give away to his ministers on a crucial exercise of the prerogative.
Nor is it in matters of routine alone that the permanent officers shape the course of colonial administration. A strong-minded minister with a policy of his own, like Lord Grey or Lord Carnarvon, will force his subordinates to carry it out, but even here a still stronger-minded under-secretary will often have his way. In 1848 Lord Grey, then Secretary for the Colonies, summoned the aged and moribund Committee (of the privy council) on Trade and Plantations to advise with him on the policy to be adopted towards the Australian colonies. The report was drafted by Sir James Stephen and we have no difficulty in discovering in its far-sighted proposals and masculine style the mind as well as the hand of the author of the essay on ‘Hildebrand.’ It is often said that a state department is inevitably wedded to routine. In the report just mentioned the striking feature is the outline of a system of Australian federation that is only now on the point of being realized. So far was the pedantic Colonial Office then, as it has often been before and since, ahead of its subject colonies.
The other colonizing countries have followed the same line of development. Beginning as direct delegations of the sovereign power to a branch, first constituent and then separated, of the sovereign’s council, the department of colonies has been in course of time made an independent ministry directly answerable to parliament. In bureaucratic France the colonies since 1854 have been associated with the navy. On the first of January, 1899, the empire on which the sun never set, having lost the last of the dependencies that were once its glory, abolished its colonial office. The sun had set on Spain to rise no more.
With the enormous progress in the arts and sciences which has characterized especially the last half of the nineteenth century, education has kept well abreast, although its progress has been gradual and it is not always easy to recognize the great advances that have been made. In the sciences, a discovery is made or a machine invented that in the course of a few years forms the basis of a new industry, gives occupation to thousands and places within the reach of almost every one conveniences previously attainable only by the few. In education no such sudden revolutions occur, and great changes are introduced by degrees without producing any commotion or any surprise. From the days of Erasmus and Rabelais, if not earlier, educational reformers have urged the importance of studying things rather than books about things, of cultivating the hand and eye as well as the mind, of training the perceptive powers, of cultivating a habit of observation and discrimination, and of developing the faculty of judgment. Yet, notwithstanding all that has been said and written, progress in this direction has until recently been very slow. Carlyle, apparently looking at the matter almost from the old scholastic standpoint, expressed the opinion that the true university of modern times was a great library; books, not things, should be studied. It would conform more to the modern point of view to say that the true university of the twentieth century is a great laboratory. Even the function of a library in our modern institutions of learning is perhaps more that of a laboratory than that of a mere storehouse of facts and opinions.
It is perhaps not too much to say that the development in the direction indicated has been greatest in our own country; that the United States have taken the lead in the revolution against the old method of teaching, and that at the present time the higher schools of this country are examples of the best practice and the highest development of the laboratory method. It may, therefore, be of interest to give the readers of this magazine a brief account of the school which has in these respects been one of the foremost, if indeed it has not led the schools of this country, the Massachusetts Institute of Technology.

With the development of the natural sciences and the growth of the constructive arts, natural science long ago gained a place in the258 curricula of the great universities of Europe; and afterwards special schools were founded for teaching the applications of science to the arts. In France, the École des Ponts et Chaussées, originally started in 1747 as a drawing school, was organized in 1760 for the training of engineers. In the States of Germany, a number of similar schools were organized early in the present century. In America, the Rensselaer Polytechnic Institute, the pioneer in technical education, was founded in 1824, and was the only school devoted to applied science until the forties, when Joseph Sheffield and Abbot Lawrence established the schools which bear their names, in connection, respectively, with Yale and Harvard. With the development of railroads, which dates from the thirties, and of manufacturing, which began in this country but a few years earlier, urgent need was felt for schools which should fit younger men to grapple with the problems which the new industries offered. These schools, however, maintained for many years but a precarious existence and were quite elementary in character. The Civil War interrupted their growth and absorbed for a time all the resources of the nation; but its termination set free an abundant store of energy, henceforward to seek its chief application in the development of trade, commerce, manufacturing and industrial pursuits of every kind. From this time the success of schools of technology was assured. They were needed to supply young men for the development of the arts; but, on the other hand, as in all things not purely material, they were to create a demand for such men by first259 furnishing a supply. Manufacturers and leaders of industrial enterprise soon found that they could not afford to do without the services of young men trained in scientific principles. In this way, by reversing the usual law of supply and demand, these schools contributed powerfully to advance the technical development of the country, far indeed beyond the measure that may be inferred from the mere number of their graduates.
The Massachusetts Institute of Technology was chartered in 1861, and first opened to students in 1865. Its claim to recognition as a leader in the development of technical education may perhaps be summarized as follows: It was the first school in the world to institute laboratory instruction in physics and chemistry to students in large classes as a part of the regular course of each candidate for a degree; the first to equip a mining and metallurgical laboratory for the instruction of students by actual treatment of ores in large quantities; the first to establish a laboratory for teaching the nature and uses of steam, and a laboratory for testing the strength of materials of construction in commercial sizes; and the first in America to establish a department of architecture. Later still, it was the first school in America to establish distinct and specialized courses of study in electrical engineering, in sanitary engineering, in chemical engineering and in naval architecture.
The success of the school has been commensurate with its progressiveness. It stands to-day the largest, most complete school of its class in the United States, and one of the largest in the world. The number of its students is 1,176, the number of its teachers, including lecturers, 175. Excluding lecturers, the number of students per teacher is only 8.7, a ratio which is a good general index of the character of the instruction. The students come from 40 States and Territories of the Union and from 12 foreign countries.
Before passing to a more detailed description of the work of its various departments, some general characteristics of the school should be mentioned. The first is the great variety of its courses and the specialization of its instruction. It is a college of general technology, embracing almost every branch of study which finds application in the arts. There are thirteen distinct courses of study: Civil and topographical engineering, mechanical engineering, mining engineering and metallurgy, architecture, chemistry, electrical engineering, biology, physics, general studies, chemical engineering, sanitary engineering, geology and naval architecture. These several departments mutually support and reinforce each other, and allow a specialization of the instruction which would be impossible in a smaller college with a less numerous staff of instructors. Thus, at the Institute of Technology, there are not only professors of civil engineering and of260 mechanical engineering, but professors of mechanism, steam engineering, railroad engineering, highway engineering, hydraulic engineering, topographical engineering, etc. Again, the chemical staff of twenty-four persons is distributed over general chemistry, analytical chemistry, organic chemistry, industrial chemistry and sanitary chemistry. There are separate laboratories for water analysis, for gas analysis, for food analysis, for dyeing and bleaching, etc. In each of these there are teachers who are able to give their entire time to instruction and research in a single line.

The second characteristic of the Institute is the predominance of laboratory, shop and field practice, experiment and research. These are used wherever it is found practicable to supplement, illustrate or emphasize the work of the recitation or lecture-room.
The third characteristic of the Institute, and one which is absent261 in the case of many similar schools, is the fact that a not inconsiderable amount of general training has from the beginning been required of every candidate for the degree. In some technical or scientific schools there are no liberalizing studies, aside from those of a professional character. The faculty of the institute have insisted that such studies should be incorporated to a considerable extent in the curriculum of every course, recognizing the fact that few students in technical schools are graduates of colleges, and that the aim of the Institute should be first of all to graduate broadly trained men. Aside from the courses in liberal studies, a broad spirit is shown in the technical courses themselves. The study of general principles is always the chief end in view, and to it are strictly subordinated the acquirement of all knacks, tricks of the trade or merely practical rules.
These characteristics of the Institute were impressed upon it from the beginning by the master hand of its founder and first president, William B. Rogers. President Rogers aimed to establish ‘a comprehensive, polytechnic college’ which should provide a ‘complete system of industrial education.’ It is now generally recognized that a complete system of industrial education would consist of three parts: First, manual training schools, for developing the eye and hand, not with the object of producing artisans, but for training alone. Second, trade schools for special training in the technique of the different trades. Third, higher technical schools for training in the fundamental principles of the sciences, and fitting men in the broadest way to become leaders in the application of the sciences to the arts. Manual training is now generally recognized as a desirable addition to every scheme of public instruction and a powerful adjunct to every technical school. It was not indicated in the original scheme of the Institute, but was added in 1877 through the wisdom of President Runkle, as a result of the exhibition in Philadelphia of the results obtained in Russia by instruction of this kind. Trade schools, for the training of artisans, were never included in the scheme of President Rogers, and are not now, either in America or Europe, considered suitable adjuncts to so-called technical schools, although they are very desirable as special and independent institutions. The original plan for the Institute contemplated simply a school of the last-named kind, together with provision for evening lectures, to which outsiders should be admitted, and which it was expected would be of benefit to artisans; and also the establishment of a museum of arts, and of a society of arts which should hold regular meetings and which should be the medium for the communication to the public of scientific discoveries and inventions. It may be as well to state here that the museum of arts was never established except in so far as the separate departments of the Institute have accumulated collections; but that the society of arts,262 which held its first meeting in 1862, has been continued to the present time. Many important inventions, as for instance the earliest forms of the Bell telephone, were first publicly exhibited at its meetings.
In outlining his plan, President Rogers showed wonderful keenness and foresight. With the added experience of the succeeding forty years, it would scarcely be possible to make a more complete statement of what experience has shown to be the best method of organization. In fact, his Scope and Plan of the School of Industrial Science of the Massachusetts Institute of Technology may be said to be the first step toward a new order of things in education, and contains the first clear statement of the desirability of teaching physics, mining, metallurgy and other branches by the laboratory method.

Let us now see what has been the result of the nearly forty years of development since President Rogers outlined his plan. Originally confined to one building, the growing needs of the school have led to the erection of five others, in addition to a gymnasium. The original building, completed in 1865, is now known as the Rogers Building, after the founder of the school; while the one next erected, in 1883, is named after the third president, the late General Francis A. Walker. These two buildings each measure about 90 by 150 feet, and in addition to a building occupied by the Boston Society of Natural History, occupy one entire square nearly in the heart of the city, and in close proximity to the Public Library and the Art Museum. Three other buildings, which adjoin each other and now form one structure, are situated about six hundred feet distant and form the front and part of one side of what will some day be one large quadrangle. The first of these buildings to be erected was the Engineering Building, built in 1889, measuring 52 by 148 feet on the ground, adjoining which is263 a building erected in 1892, 58 by 68 feet on the ground, and now forming part of the Engineering Building. Adjoining this is the Henry L. Pierce Building, erected in 1898, and measuring 58 by 160 feet. In addition to these buildings are the workshops, about a quarter of a mile distant, covering 24,000 square feet, and a gymnasium and drill hall.
The first laboratory to be established at the institute was that of chemistry, and this leads us to speak first of the department of chemistry. The laboratory of general chemistry was opened in 1876 under the direction of Professors EliotB and Storer, and is believed to be the first laboratory where instruction was given in general chemistry to classes of considerable size. From small beginnings, this department has rapidly grown under the able direction of such men as James M. Crafts, (since 1897 president of the Institute), William Ripley Nichols, Charles H. Wing, Lewis M. Norton and Thomas M. Drown, until now the instructing force consists of five professors, thirteen instructors and six assistants, a total teaching force of twenty-four, in addition to seven or eight lecturers on chemical subjects. The department occupies the two upper floors in the Walker Building, together with about half of one floor in the Henry L. Pierce Building, devoted to industrial chemistry. The laboratories, which are said to be the264 largest and best equipped in the United States, are known as the Kidder chemical laboratories, having been so named in recognition of the generosity of the late Jerome S. Kidder. They comprise twenty-two separate laboratories, three lecture-rooms, a reading-room and library, two balance-rooms, offices and supply-rooms, making forty rooms in all, with accommodation for seven hundred students. Besides the large laboratories for general chemistry and analytical chemistry, there are smaller laboratories for volumetric analysis, for organic chemistry, for sanitary chemistry with special reference to the analysis of water and air, for oil and gas analysis, for the optical and chemical examination of sugars, starches, etc., for the determination of molecular weights, and so on. In the industrial laboratories, the students are taught how to manufacture chemicals with due regard to economy of material, space and time. There is also a special laboratory for textile coloring, with printing machines and all the necessary equipment of baths, dryers, etc., for experimental dyeing and coloring. In this laboratory the preparation and use of coloring matters are taught with the object of fitting young men for positions in dye works. A course of lectures in textile coloring was first introduced in 1888 and the laboratory course in 1889.
B Now President Eliot of Harvard.
A large amount of original work is accomplished each year in these laboratories, both by students and professors. During the year 1897–98, for instance, four books and sixteen articles on chemical subjects came from them. In the development of sanitary chemistry the Institute has been particularly prominent. Beginning with the careful and thorough investigations made by Professor Nichols for the State Board of Health, the reputation of the institute in this direction has been still further increased by the recent extensive investigations of Professor Drown and Mrs. Ellen H. Richards, made for the same board in connection with the examination of the purity of the water supplies of the State, and the experiments at Lawrence relating to the best methods for purifying water and disposing of the sewage of inland towns.
An illustration of the policy of the school in separating out a subject whenever it is found capable of complete theoretical and practical treatment and putting it into the hands of some assistant professor for development, is found in the laboratory for gas and oil analysis, which for some years has been in charge of Dr. Gill. In this laboratory, investigations are made relating to chimney gases, as well as questions of fuel, furnaces, gas firing, etc., while oils are tested and analyzed with reference to specific gravity, viscosity, friction, flashing and firing points, and liability to spontaneous combustion. The same policy is further illustrated in the establishment in 1894 of a well equipped laboratory devoted entirely to physical chemistry; that is to say, to the relations between chemical changes and heat, light and electricity.265 This laboratory, under the charge of Dr. H. M. Goodwin, occupies a room measuring 28 by 29½ feet, and is devoted to photographic work, experiments in electrical conductivity, thermo-chemistry, molecular weight determinations and experiments in chemical dynamics. More recently still, a complete option in electro-chemistry has been established, to meet a growing demand.
Still another illustration of the policy of specialization is afforded by the action of the Institute in establishing new courses of study, extending through the entire four years, whenever the need is felt for men trained in a direction not hitherto specially provided for. Thus, in 1888 a new course was established in chemical engineering. The chemical engineer is not primarily a chemist, but a mechanical engineer—one, however, who has given special attention to such problems as the construction of dye works and bleacheries, sugar refineries, soap works, paper and pulp manufactories, fertilizer works, chemical works, and in general all the problems of chemical machinery and manufacturing. That this new course filled a real want was soon made evident. The first class, that of ’91, contained seven graduates, while eighty-eight students in all have now been graduated and are for the most part engaged in chemical works.
The physical laboratories of the Institute are now known as the Rogers laboratories. Although they formed perhaps the central feature of President Rogers’ plan, financial and other exigencies prevented their being established when the school was opened. In 1869, Prof.266 Edward C. Pickering, then in charge of the department of physics, submitted a scheme to the government of the Institute entitled ‘Plan of the Physical Laboratory.’ This plan was adopted and carried out in the autumn of 1869 and has been in use ever since. It is worthy of remark that the original statement of Professor Rogers with reference to laboratory instruction in physics contained no mention of electricity, then a subordinate branch, but one whose development since has caused it to occupy the leading place in any physical department. In 1882 the corporation established a course in electrical engineering, setting an example which has since been followed by almost every large technical school, and founding a course destined in a few years to become one of the largest in the Institute.
At present the department of physics and electrical engineering, under the head of Prof. Charles R. Cross, has an active teaching force of one professor, four assistant professors, six instructors and three assistants, a total of fourteen. In addition to these, there are twelve lecturers on special topics, including many men eminent in their profession. The Rogers laboratories occupy sixteen rooms in the Walker Building, including two lecture-rooms and ten laboratories. As in the case of the chemical department, these laboratories are highly specialized. There is a laboratory for general physics, one for electrical measurements, two rooms devoted to a laboratory for electrical engineering, containing two distinct power plants driven by steam engines of 100 and 150 horse-power, with a large number of dynamo machines, transformers and a great variety of other apparatus arranged for purposes of instruction, the mere enumeration of which would occupy several pages. Moreover, a lighting and power plant in the new building on Trinity Place is available for experiments and instruction. Besides these, there are rooms for photometry, for heat measurements, for acoustics, for optics and for photography. In fact, probably no department of the Institute is more fully equipped than this, the wealth of apparatus being so great that the casual visitor is confused by the network of wires and machinery which surround him.
The interdependent and harmonious work of the various departments of the Institute is shown in the development of special lecture and laboratory courses, and is in marked contrast to the policy of departmental isolation sometimes practiced. Thus, in 1889, two new courses of instruction were established by the physical department in response to the demand of the department of mining; namely, the course in heat measurements, including measurements of high temperatures, the determination of the calorific power of fuels, etc., and a course on the applications of electro-metallurgy to chemical analysis, the reduction of ores and similar problems. The equipment of calorimeters, pyrometers, etc., in the heat laboratory is said to be so large267 as to permit a more complete examination of the efficiency of fuels than has hitherto been possible anywhere.
Perhaps the greatest innovation made by the Institute in the early days was in establishing a laboratory for the teaching of mining and metallurgy. Previous to 1871 metallurgical work was done in the chemical laboratories, but in that year the mining and metallurgical laboratory was put into operation through the efforts of President Runkle, Professor Richards and Professor Ordway. Prior to this date, there were assaying or metallurgical laboratories at the École des Mines at Paris, the Royal School of Mines in London, the German Mining Schools at Freiberg and Clausthal and Berlin, and also in several technical schools in this country. The German mining schools were situated beside smelting works, but the plants could not often be used for experiments by professors or students in a way to alter the usual method of running. In all these laboratories, however, the apparatus was designed to treat quantities of ore not exceeding a few ounces for each test. The Institute laboratories were the first in the world which268 were designed for the treatment of ores in economical quantities of from five hundred pounds to three tons, and used entirely for purposes of instruction. They are now known as the John Cummings laboratories, in memory of one who for many years was treasurer of the Institute and one of its most devoted friends. They now occupy the entire basement of the Rogers Building, and include laboratories for milling, concentrating and smelting ores, as well as for testing them by assay and by blowpipe. The development of these laboratories from the small beginnings of 1871 has been mainly due to the efforts of Prof. R. H. Richards, past president of the American Institute of Mining Engineers, whose contributions on methods of ore dressing are well known to mining engineers. The staff of this department also includes Prof. H. O. Hofman, well known for his researches in metallurgy.
Mention should here be made of the department of geology, which is under the direction of Professors Niles, Crosby and Barton, and which now occupies commodious quarters comprising the greater part269 of a floor in the Henry L. Pierce Building. The collections of this department number many thousands, and are supplemented by those of the Society of Natural History, which are available for purposes of instruction. As would be expected in a school of applied science, the economic aspects of geology are kept closely in view, and the work is adapted to the particular object to be attained. The student in architecture, for instance, receives a course in geology in which the study of building stones is a prominent feature.
An engineering laboratory formed part of the original scheme of President Rogers, although he included it under the head of physics and did not anticipate the importance which has since attached to it. Such a laboratory, especially devoted to engineering, was established on a small scale in 1874, through the efforts of Professor Whitaker. An engine for experimental purposes was presented to the institute by Mr. G. B. Dixwell, and this, with other apparatus, constituted what is believed to have been the first engineering laboratory in the world for the regular instruction of classes. For lack of funds and space, it was not much developed until 1882, but since that time it has been brought to a high state of efficiency. To-day the engineering laboratories, as they are called, which include laboratories of steam engineering, hydraulics, for the testing of materials and a room containing cotton machinery, occupy a floor space of 21,380 square feet on the two lower floors of the Engineering and Pierce Buildings. In addition to this, there are workshops which will be referred to again. It would be tedious to enumerate the great variety of apparatus to be found in these laboratories, but a few important points may be mentioned. In the steam laboratory a 150 horse-power triple-expansion Corliss engine, the first of its kind of practical size ever arranged for experimental purposes, was purchased in 1890 and is regularly used for testing purposes. A second engine of 225 horse-power was added two years ago, transferring its power through a rope drive. Besides these two large engines, there are a number of smaller ones for experimental purposes and the study of valve setting, and, in addition, there are gas engines, hot-air engines and other apparatus. There is also a collection of cotton machinery sufficient to make clear to the student the mechanism of the various machines.
The hydraulic laboratory is well equipped for the study of the laws of flowing water, having a steel tank five feet in diameter and twenty-seven feet high, with a system of stand-pipes eighty-five feet high, reaching to the top of the building. This tank is furnished with gates and other apparatus suitable for experiments on the flow from orifices, and connected with a system of horizontal pipes by which a large variety of other investigations may be carried on. Among the other apparatus of interest may be mentioned two impact water wheels,270 placed in housings with glass sides so that the action of the water on striking the buckets can be observed.
Some experiments have already been made in the laboratory on the flow of air, the results of which have been communicated by Professor Peabody to the American Society of Mechanical Engineers. It is now intended to continue the study of the flow of air and its use as a motive power in great detail, just as the flow of water is studied, and an air compressor of 100 horse-power, which will produce a pressure of twenty-five hundred pounds, is now being installed.
The laboratory for testing the strength of materials was established in 1881 by Prof. G. Lanza, and has since been extensively developed under his direction, until it is now one of the most complete in the world. It is perhaps not too much to say that the experiments made in this laboratory have in some respects revolutionized the ideas of engineers. Previous to its establishment, the only tests of timber that had been made were upon small selected specimens one or two inches square and a few feet long. The results of these tests had been used for years by architects and engineers, and they were given in all the engineering handbooks. In the Institute laboratory there were conducted the first systematic and extended tests of beams of commercial size. The results soon showed that the strength of such timber was a271 great deal less than previous tests on small beams had indicated, and the practice of engineers and architects has since that time been completely modified through the results obtained in this and similar laboratories. In this way does the work of such a laboratory become of direct and lasting value to the arts. The central piece of apparatus of the Institute laboratory is the Emery machine, similar to the great machine at the Watertown arsenal, with a capacity of three hundred thousand pounds. But in addition to this machine there are a dozen or more other machines designed to test beams, columns, rope, wire and, in fact, materials of every kind and in every form. An interesting machine is that for testing shafts in torsion, and it is instructive to see it twist off with apparent ease a steel shaft three inches in diameter, twisting the fibers before they break till the rod resembles a barber’s pole. There are also beam-testing machines with capacities up to one hundred thousand pounds, in which not only beams but wooden trusses may be tested to the breaking point. Some of the apparatus is of great delicacy; for instance, one instrument will measure the twist of a steel shaft two and a half inches in diameter and six feet long so delicately that the effect of a twist given by one’s hand is distinctly visible; scientifically speaking, it will measure an angle of twist of two seconds. There is also a machine designed for testing stone arches, having a capacity of four hundred thousand pounds and suitable for an investigation of272 many questions concerning these uncertain structures; also machinery for studying the wear of brake shoes and wheel tires, a subject in regard to which there is room for much investigation. Finally, mention should be made of machinery for investigating the interesting subject of the effect of repetition of stress.
The tests performed in the engineering laboratory cover almost the entire range of mechanical science. Sometimes investigations are carried on through a number of years; for instance, during three successive years experiments were conducted and formed the subject of theses on the proper method of counterbalancing the reciprocating parts of a locomotive. Nor are the tests performed by the Institute students as a regular part of their instruction confined to these laboratories, as is made evident by the fifty-hour test of the West End Street Railway power station and the twenty-four hour test of the pumping engine at Chestnut Hill, both recently carried out.
In connection with the engineering laboratories, brief mention may be made of the shops, which form an important adjunct of the laboratories. They consist of a shop for carpentry, wood-turning and pattern-making, equipped with forty carpenters’ benches, thirty-six pattern-makers’ benches and a full equipment of saws, planers, lathes, etc.; a foundry with a cupola furnace for melting iron, thirty-two moulders’273 benches, two brass furnaces and a core-oven; a forge shop with thirty-two forges, a power hammer, vises, etc.; a machine shop with about forty lathes, together with drills, planers and all the other necessary apparatus used in machine tool work.
The magnitude of the Institute laboratories is shown by the following statements: The total horse-power of steam and other engines is nine hundred and eighty-three; the total capacity of tension, compression and transverse testing machines is over eight hundred thousand pounds, and of torsion testing machines about one hundred and fifty-six thousand inch pounds; the total horse-power of hydraulic motors is sixty-two; and the total capacity of pumps is thirty-two hundred gallons per minute.
The engineering laboratories are used by students of all the engineering departments, that is to say, by a large majority of the students in the school. The benefit derived by this actual contact with materials and with machines of commercial size, under proper instruction, is believed to be very great.
The department of mechanical engineering, one of the original departments, is now the largest in the school, having a force of instruction of five professors and twelve instructors and assistants. As an offshoot of it, a department of naval architecture was established in 1893, after a preliminary experience of four years with an option in this direction. This was the first course of its kind established in this country. It is somewhat remarkable, considering the preëminence that America has long enjoyed in the building of ships and marine engines, that our technical schools should for so long have failed to offer specialized instruction in these important branches. Schools devoted to these subjects have long existed abroad. The French Government School of Naval Architecture was established in 1865 for the purpose of educating young men for the Government service. To this school foreigners are admitted under certain restrictions. In England the first school of naval architecture was opened in 1871, but no systematic instruction seems to have been provided until 1864. At present, however, the Royal Naval College, at Greenwich, gives excellent and thorough instruction to young men desiring to enter the Government service. There has also been for a number of years an excellent course of study in naval architecture at the University of Glasgow. The Institute of Technology established in 1888 an elementary course in ship construction, and this was followed in 1890 by a specialized option in naval architecture extending through the four years. Already forty-one men have graduated from this course.

One of the large departments of the school is that of architecture. Forming one of the original departments established at the beginning of the Institute in 1865, when there was no similar department in this274 country, it may fairly be affirmed to have led in the development of instruction in this important profession. It was for many years in charge of Prof. W. R. Ware, who left the Institute in 1880 to assume charge of the newly established department at Columbia College. In common with the other departments of the Institute, that of architecture has developed enormously within recent years. Three times since 1883 has the department been obliged to change its location in order to meet the continued need of expansion. From the original small quarters in the upper floor of the Rogers Building, it has grown so that it now occupies two and one half floors in the Pierce Building, besides a large room for modelling in another building. The drawing-rooms now accommodate over two hundred students. The department has a magnificent library and a very large collection of photographs and lantern slides. Under the careful management of275 Prof. F. W. Chandler, who at the same time is head of the Architectural Department of the city and member of the Fine Arts Commission, it has now attained a most enviable reputation. Institute students competed for several years for the prizes offered by the New York Société des Beaux Arts, and in each competition in which they entered they carried off the gold medal and the highest honors. In the three competitions of ’94–’95, no less than seventy sets of drawings were submitted by all competitors. The two gold medals, four first mentions and two second mentions were awarded to Institute students. Of the nine designs sent from the Institute, six were placed by the jury among the first eight of the seventy designs submitted; two received second place and one was put out of competition because of too great deviation from the preliminary sketch. This great success is doubtless due to the rigorous training which the students receive in architectural design at the hands of Professor Despradelle, himself a graduate of the École des Beaux Arts, a winner of high honors in Paris, and of the third prize in the recent Phœbe Hearst world competition for the new buildings of the University of California, and within a few weeks the winner of the first medal in architecture in the Paris Salon of 1900. For three years the students are continually engaged upon architectural design, and the work of each student is examined and criticised before the class by a jury from the Boston Society of Architects. Students in architecture have also the opportunity, if they desire, of taking an option in architectural engineering, in which they are given a course in the theory and design of structures as rigid as that received by the students in civil engineering. The relations between architecture and engineering are exceedingly close and are becoming closer every year. The work of the architect, aside from the æsthetic design of his buildings, is becoming more and more like the work of the engineer, and requires a thorough knowledge of engineering construction.
During the past year, after very careful consideration, the faculty has also established an option in the course of architecture, devoted particularly to landscape architecture, including, besides a large amount of work in architecture proper, instruction in horticulture and landscape design, on the one hand, and in surveying, topographical drawing, drainage, etc., on the other hand. The landscape architect has heretofore had no opportunity to secure a thorough training in his profession, except by passing through an apprenticeship, as was formerly necessary in the older professions. On account of the steady increase in this country in the demand for trained landscape architects and the increasing attention which is now being paid by our municipalities to questions concerning public parks, and also by private individuals to the beautifying of private grounds, there seems now to be an unusual opportunity for young men to devote themselves to this branch of276 the profession. As usual, the Institute of Technology is early in the field with a course designed to this end.

The last of the engineering departments to be considered and one of the largest, is that of civil engineering, a department established when the Institute was founded, and until 1881 under the direction of that accomplished scholar and teacher, Prof. J. B. Henck, and since 1887 in charge of the writer. This department has grown since 1886 from four to eleven teachers, and from sixty to one hundred and fifty-three students in the three upper classes. It now occupies the two upper floors of the Engineering Building, or about twenty-three thousand square feet. In recognition of the increasing importance of sanitary questions affecting the health of communities, a new branch of civil engineering was recognized by the Institute in 1889 by the establishment of a regular four years’ course in sanitary engineering, in which particular attention is directed to such problems, and students are afforded opportunities of studying the bearing of chemistry and biology upon them. Here again the breadth and specialization of the work at the Institute was shown, rendering it possible with no change in the teaching force and with no disarrangement of studies, to establish such a course of instruction as soon as the need for it became apparent.
Interesting work has been done under the direction of Professor Burton, professor of topographical engineering, in connection with the277 measurement of base lines with the steel tape. After devising an apparatus for holding and supporting the tape, and measuring the coefficient of expansion of actual tapes, an application was recently made of the thermophone for determining the exact average temperature of the tape. This instrument, which was invented a few years ago by two Institute graduates, allows the average temperature of the tape to be measured within half a degree.
An interesting department of the Institute, and one that has of recent years assumed great practical importance, is that of biology. It was organized in 1882, as an outgrowth of what was prior to that date the course in natural history, and now has a teaching force of six, under the direction of Prof. William T. Sedgwick, and occupies, with its laboratories and lecture-rooms, one entire floor of the Pierce Building. There are five distinct laboratories, fully equipped, with private rooms, store and preparation rooms, and a library and reading-room, and it is perhaps safe to say that nowhere in the United States is there so compact or well arranged a series of laboratories devoted chiefly to the sanitary, hygienic and industrial aspects of biology. The great advances in sanitary science in recent years have made bacteriology one of the most important, as well as one of the most practical, of the biological sciences, and the biologist has taken his place beside the chemist and the engineer in the study of the science and art of public sanitation. But bacteriology is of importance, not only in sanitary science, but also in its industrial relations. Great industries, like those connected with food preserving, canning, vinegar making, tanning and brewing, depend upon the activity or the exclusion of micro-organisms. As might be expected in a school of applied science, the development of the biological department in the Institute has been mainly along sanitary and industrial lines, rather than in the direction of zoölogy. The biological work in connection with the recent important investigations of the State Board of Health regarding the purification of water and the disposal of sewage, was done at the Institute, and early led to special instruction in these directions. In 1894 a course was established in the micro-organisms of fermentation, not only new to the Institute, but, it is believed, to the United States. Important researches had been made in Denmark in these lines, and in order to become thoroughly familiar with them, one of the instructors of the department spent a summer in the laboratory of Alfred Jörgensen, in Copenhagen. In 1896, a more elaborate course, that in industrial biology, was established, and since that time special studies have been made in various lines, such as the efficiency of sterilizing processes, the preparation of canned goods and the cultivation of butter bacteria. This department is destined to still greater development in the near future, and its laboratories are finely equipped in every direction.

Reference to the different departments in the Institute would not be complete without brief mention of its department of general studies. It is perhaps seldom recognized, but it is nevertheless a fact that the Institute, although primarily a technical school, is better equipped for giving instruction in languages, in history, in economics and statistics and in political science than many classical institutions. Indeed, the only important department of study which is found in such institutions, and for which no provision is made at the Institute, is that of ancient languages. The force of instruction in the department of general studies, leaving out of consideration the department of modern languages, comprises two professors, one associate professor, three assistant professors, one instructor and one assistant, a total of eight, probably a larger number than is found in any but the very largest colleges. In the department of modern languages, there is one professor, one279 associate professor, one assistant professor and four instructors. There are offered ten distinct courses in English, eleven in modern languages, eight in history and twenty in economics and statistics and in political science. As already stated, it has been a fundamental principle in the government of the school that all regular students should receive a not inconsiderable amount of instruction in these subjects, but in addition to the engineering and other technical courses, there is a so-called course in general studies, designed to train young men for business occupations, in which, besides thorough courses in chemistry, physics and other sciences, a large amount of time is devoted to the general studies which have been referred to. The late president of the Institute, General Walker, whose principal work, aside from that relating to education, lay in the field of economics and statistics, took great interest in the development of this general course, and to him, more than to anybody else, is due its present high standard. Seventy-eight young men have graduated from the department, and in many respects its course of study offers advantages over the usual college course.
Summer schools are maintained by the Institute in the departments of civil engineering, mining engineering and architecture. That in civil engineering affords continuous field practice in geodesy and hydraulics during about a month. That in mining engineering affords students an opportunity to visit mining or metallurgical works and to become practically acquainted with the methods employed by actually taking part in them. These summer schools in mining and metallurgy have been held in all parts of the country, from Nova Scotia to Lake Superior and Colorado. The summer school in architecture consists not infrequently of a trip abroad, with detailed studies and sketches of special types of architecture.
The Institute also offers extended courses of free evening lectures, of which twenty courses of twelve lectures each were given during the past year. These courses, established by the trustee of the Lowell Institute under the supervision of the Institute, correspond to one portion of President Rogers’s original plan, and are fully appreciated by young men who cannot afford the time for a complete and consecutive education. The trustee of the Lowell Institute also established in 1872, and has maintained ever since, a special school of practical design, under the supervision of the Institute, in which young men and women are given free instruction in the art of making patterns for prints, ginghams, silks, laces, paper hangings, carpets, etc.; the object being to fit them to engage in the textile industries especially, but also in other branches of manufacture in which taste in form and color is an essential element for success.
Mention may be made here of the fact that all work at the Institute is open to women on the same terms as to men. As early as 1867,280 among the Lowell free courses, there were two chemical courses open to both sexes, and soon afterward women were admitted to the regular work of the school. The first woman to graduate was Mrs. Ellen H. Richards, in 1873, and since that time forty-eight women have received the degree. This number, however, is no measure of the part which women have taken in the work of the school, for a large majority of those who attend are special students. During the year 1899–1900, there were fifty-three women studying at the school, principally in the departments of chemistry, biology, geology, physics and architecture. From the last-named course eleven young women have graduated, one of whom was the designer of the Woman’s Building at the Chicago Exposition.
One peculiarity of the Institute which has not been mentioned is the sub-division of its libraries. Instead of having one general library, each department has its special library, conveniently located with reference to its rooms. This involves a slight duplication of books, but is of the greatest advantage to students and teachers for consultation. The Institute libraries are not large, compared with the libraries of many colleges and universities, but they are remarkably rich along the lines of the special topics to which they are of necessity principally devoted, and particularly in scientific periodicals. The total number of periodicals in all languages regularly received at the Institute, not including a large number of official reports, is eight hundred and forty-seven. In the engineering library alone there are one hundred and seventy-three. It is believed that this forms one of the largest collections of scientific journals to be found anywhere. The Institute publishes a scientific magazine, known as the Technology Quarterly, which was established in 1887, and is the official organ for the publication of the results of tests in the laboratories and of special investigations by members of the staff and by students and alumni. The Association of Class Secretaries also publishes the Technology Review, a more popular quarterly, established only two years ago, and devoted to the social and general interests of the Institute. In 1896 the Technology Club was started, occupying a building near the Institute and affording alumni and students the social advantages of a clubhouse. The alumni of the Institute now number two thousand three hundred and thirty-nine; they maintain an Alumni Association which holds annual meetings, and seven local branch associations which are scattered over the country from the Connecticut Valley to Colorado.

In reviewing the success which this school has attained, the question naturally presents itself: To what is this success due? Let me here record my conviction that it has been due mainly to the courage and devotion of its corporation and of the presidents who have directed its policy. In this respect no institution was ever more fortunate. With281 a guiding body possessed of the courage and faith that have animated the corporation of the institution from the earliest days, and especially with the able men who have been its presidents, success was assured. While the school was yet struggling for its very existence, with few friends and little money, they never faltered. They have not hesitated again and again to plunge the school deeply into debt when its needs required it, trusting to the generosity of New England that it should not be allowed to be crippled, and each time has their confidence been justified. Poverty has never been permitted to impair the efficiency of the school. As President Crafts remarked in a recent annual report, “We are less favored than many neighboring institutions in building space, but we have always followed the wise policy of keeping in the foremost rank and in some departments leading the way in supplying the best methods and apparatus for teaching and for making investigation. We have run in debt to buy them, and run282 still further in debt to build houses to hold them, but we have always had them when the head of a department told the government of the school that they were necessary to the most efficient teaching of his science.” With a corporation acting on such a principle there could be no failure. It is true that the faculty have stood unfalteringly, even in the darkest days, for high scholarship; and equally true that the school has been remarkably fortunate in the character of the young men who have sought its halls, but no faculty and no body of students could have brought success with a corporation less broadminded and courageous. Let me here add my tribute to the work which was done by the late General Francis A. Walker, president of the institute from 1881 till 1897. Probably no single person did more to secure the success of the school than he. His great administrative ability, his wide acquaintance, his accurate judgment of men, his magnificent courage and his splendid enthusiasm, were factors in the development of the school whose importance it is difficult to overestimate.
General Walker was succeeded by President James M. Crafts, who had been connected with the Institute for many years as professor of chemistry, and under whose energetic administration the progress of the Institute has been steadily continued. In fact, thanks to some unexpected additions to the funds of the school, its material resources and its equipment have been more enlarged and extended during the past three years than in many years previous. Only a few months ago, however, President Crafts, desiring to devote himself more uninterruptedly to the pursuit of the science which first awakened his enthusiasm and in which he has attained such eminent distinction, both in this country and abroad, decided to relinquish his office. The corporation has chosen as his successor, Dr. Henry S. Pritchett, for many years professor of astronomy in Washington University, St. Louis, and for the past few years superintendent of the Coast and Geodetic Survey at Washington. A more fortunate selection could not have been made, and the well-known scientific and administrative ability of Dr. Pritchett will no doubt be the means not only of maintaining the present high reputation of the school, but of extending and enlarging it.
Unfortunately, the Institute is still unendowed in the sense that its receipts from invested property constitute but a very small part of the means required to carry on the school. To quote from one of President Walker’s reports, “No other institution of our size but has two, three or four times the amount of wealth to draw upon which we possess. It has only been exceeding good fortune, combined with extreme courage, energy and self-devotion on the part of its trustees and teachers that has more than once rescued the school from paralysis, if not from extinction.” In 1898–’99, the total expenditures of the school were about $367,500, while the current receipts were about $347,500, showing283 a deficit of about $20,000. Of the current receipts, $207,000, or 59 per cent, were from students’ fees. Dividing the total expenditures by the number of students, we find an expenditure of $314 per student, without counting interest on the value of land and buildings, while the tuition fee is $200. The invested funds of the Institute amount to but $1,917,000. All gifts and legacies, with the exception of this amount, have had to go into land, buildings and equipment. Between 1888 and 1899 the Institute has been obliged to spend $350,000 for land, the purchase of which has been a great burden, and within a few years a further expenditure of $260,000 in this direction has been made.

The bearing of these figures will perhaps be realized by comparing them with similar figures regarding Cornell University, which is largely a technical school, since nearly one half of its students pursue technical284 courses similar to those in the Institute. In 1898 the total income of that university was $583,000, of which about $121,000, or only 20 per cent, was received from tuition fees. Its invested funds amounted to $6,446,818.
The State has generously given aid to the Institute in some of its most trying times; as in 1888, when it gave $200,000, one half unconditionally and the other half for the support of free scholarships; and again in 1895, when it granted unconditionally $25,000 a year for six years and $2,000 a year additional for scholarships. Although the school has a very inadequate endowment, yet the future looks bright. It is significant of the general appreciation of its work that men and women who have not received a technical education have devoted a large part of their fortunes to providing such education for others. Among the recent benefactors of the Institute we may name Henry L. Pierce, John W. Randall, Mrs. Julia B. Huntington James and Edward Austin, who, within less than three years have bequeathed nearly a million and a half dollars to the school. If the large gifts of recent years are continued, the school will before long be put financially upon a level with its neighbors. May we not hope that as the applications of science to the arts enrich the alumni and friends of the Institute, they may help to make the road easy for their successors by devoting a part of their riches to the advancement of technical education?
A well-known Washington newspaper correspondent, writing of the recent Congress of the Daughters of the American Revolution and its disorderly meetings, says: “It is the unanimous opinion of those who have attended the congress that, while the Daughters of the American Revolution, individually, are nearly all intellectual, refined and attractive women, collectively they are an uncontrollable mob.” Why is the social conduct of human beings different from their conduct as individuals? This is the problem of the new science of social psychology. The following study of crazes and epidemics is offered as a slight contribution to this science.
By way of preface it might be said that a good deal of the confusion as to the subject matter of social psychology would be avoided if it were understood that this science is not the study of any mysterious entity called ‘the social mind,’ nor the mere study of those individual traits that make men social beings, such as imitation and suggestibility; but rather the study of the peculiar and characteristic behavior of the mind of the individual when under the influence of the social afflatus. Under this influence we do indeed find that he becomes a different being, and that his mental processes must be formulated by different laws; and we are convinced that, as thus understood, social psychology is just as distinct and legitimate a branch of study as is the psychology of the child or the psychology of sex.
Now, in what ways is the behavior of man as a social being different from his behavior as an individual? To answer this question in part, let us examine his behavior in mental epidemics and crazes. I select these because they illustrate in somewhat extreme form the influence of the social afflatus.
If, for the sake of comparison, we first consider the normal individual as such, we find that he is a perceiving, remembering, associating, judging, reflecting, reasoning being; that he is subject to certain feelings, emotions, desires and impulses, prompting him to action; that his action is more or less deliberative, and, when it finally occurs, is the result of a set of motives determined by the man’s character, which in turn is the outcome of his heredity and education and his general ability to appreciate and reflect the moral ideals of the social order to286 which he belongs. If now we study this man in respect to his mental development, whether from the savage or the child, we find that the direction of change has been away from imitative, impulsive action, towards thought, reflection, deliberation. He continually makes more use of memory and, anticipating the future, regulates his action in the light of his past experience. This change from the imitative and impulsive to the reasoning man accompanies the development of the higher brain centers, particularly of the cerebral cortex, upon which depend the all-important functions of memory and association. As an experiment it is quite possible to reduce this highly developed reasoning being in a single moment to a condition resembling his primitive state by means of hypnotism. In hypnosis there is a temporary paralysis or sleep of the higher brain centers, upon which depends deliberative, rational action, and, the lower (older) centers alone being active, the subject becomes a mere ideo-motor machine acting out every suggestion. In various related states of automatism, where there is any spontaneity at all, the mentality and morality of the subject are of a lower type and may be called reversionary in character, owing, no doubt, to the fact that those brain centers which represent the most recent acquirements of the race are temporarily out of the circuit.
If again we study the mind of the child, we find that it presents many points of likeness to the mind of the hypnotic subject and to the mind of the primitive man. We learn from biology that the child is to some extent a recapitulation of the life of the race, passing through in his individual development the stages of race development. Physiologically speaking, the higher brain centers and the centers for association, which are late acquirements of the race, are last developed in the child. We are therefore not surprised to find that the child, like the savage and the hypnotic subject, is imitative, impulsive, non-reflective, incapable of much abstract thought, deliberation or reasoning, and that he acts with a view to immediate rather than remote ends.
If now we turn to the behavior of the normal adult man in mental epidemics and crazes of all kinds, from the Crusades to the Massacre of St. Bartholomew, from the tulip mania in Holland to the Dewey welcome in New York City, we observe that his behavior is to some extent similar to that of the hypnotic subject, and the child, and the primitive man. The general character of mental action in epidemics is as follows: Men become imitative beings and their actions are determined by suggestion from the actions of others. Memory and the association of ideas are inactive, and there is an inability to reason and an indisposition towards deliberation and calm reflection. Past experiences are disregarded, remote consequences are not seen and behavior is impulsive and spasmodic. Feeling is very strong and every kind of emotion is apt to be exaggerated. Calm observation is also lacking and287 mental images may be mistaken for objective reality, as in the case of the hallucinations that are frequent in these phenomena.
The moral peculiarities of an epidemic are of a similar kind. Under the influence of a craze, the moral character of a people suffers a reversion to a primitive type. In times of epidemic waves the moral standards of the crowd approach those of the savage. We observe the exhibition of primitive instincts, such as cruelty, revenge and blood-thirstiness, together with changeableness, fanaticism, self-sacrifice and enthusiastic devotion to a leader. All these moral traits were well illustrated in the Revolution crazes in France and in the persecution of witches in the sixteenth and seventeenth centuries. Even in our own times a striking example of the primitive character of the morality of a people under the influence of social excitement was seen in the battle-cry of our American sailors in the recent Spanish war, ‘Remember the Maine,’ the ethical motive being a precipitate impulse to seek revenge. An instance like this can not be explained upon the theory that it represented the actual individual morality of the sailors participating in the battles, for it was echoed and apparently endorsed by the press throughout the country and upon the platform and even in the pulpit. It is hardly conceivable that an Englishman of noble birth should openly boast of his joy in being revenged upon an enemy; yet collective England is wild with delight when ‘Majuba Hill is avenged!’
We are thus led apparently to the theory that, for some reason not yet evident, under the influence of social excitement, something takes place in the brain of the individual not unlike the action of hypnotism, by which the higher centers representing the more recent moral and mental acquirements of the race are temporarily paralyzed, reducing the subject in a greater or less degree to the condition of the child and of the primitive man. The observation of certain physical phenomena which often accompany mental epidemics tends to confirm this theory and at the same time to suggest a possible explanation. Epidemics of the more extreme kind are apt to be accompanied by great muscular excitability, varying all the way from mere extreme mobility, such as shouting, jumping and throwing the arms, to convulsions like those of epilepsy. The dancing manias of the fourteenth and fifteenth centuries furnish the best illustrations of this, although these phenomena did not equal in intensity the frightful physical convulsions during the religious revivals in Kentucky at the beginning of this century. The particular character of these muscular movements is determined by imitation and suggestion. The movements themselves are no doubt due to congestion and irritation of the motor centers, or at least to a rapid overflow of nervous discharges at these centers, an accompaniment of the excessive emotion which attends all mental epidemics. In such a condition of the nervous system, thought, reasoning, memory and288 association can have little place, or, to express it physiologically, the unusual excitement in the lower centers of the brain accompanying excessive emotion may not only find expression in muscular movements, but may also exercise an inhibitory or paralyzing effect upon the higher centers, resulting in a kind of hypnotic condition. Neither is it difficult to understand the presence of this excessive emotion during mental epidemics or during any purely social movements, when we remember that war itself is the great original social movement, which even in this age always takes the form of a mental epidemic called the war spirit. The emotional effect of the mere physical congregation of a large number of men, the emotion increasing with the size of the assemblage, is known to all.
As we glance now at a few of the typical mental epidemics of history, we shall notice the ever-recurring presence of some or all of the mental and moral traits that I have pointed out. For illustrations of these phenomena we may turn indifferently to ancient, medieval or modern history. They abound at every period.
Very good examples may be found in Hecker’s ‘Epidemics of the Middle Ages.’ In the Crusades, particularly in the Children’s Crusades, we may observe all the mental, moral and physical peculiarities that have been mentioned. In the anti-Semitic mania, we see in its history of criminal horror the dehumanizing effects of the epidemic and the moral reversion which takes place under the influence of social excitement. The peculiar physical phenomena which have been referred to as characterizing epidemic excitement are best illustrated in the dancing manias of the Middle Ages and in the religious revival. Although epidemic ‘revivals’ have occurred in all countries, some of the best illustrations are seen in America in its early history and to some extent at the present day. At the time of the elder Edwards, revivals were accompanied by fainting, falling, tremor and numbness. In the Kentucky revivals the meetings, called camp meetings, were held in the open air. The interest in them spread in true epidemic form. At the height of the excitement, as many as 20,000 people, men, women and children, were gathered in a single camp at one time. Dr. Davidson, who writes a history of this revival, says that “the laborer quitted his task, age snatched his crutch, youth forgot his pastime, the plough was left in the furrow, business of all kinds was suspended, bold hunters and sober matrons, young men, maidens and little children flocked to the common center of attraction.” The emotional tension was very great. A boy perhaps would spring to his feet and begin to rave, or some over-excited person would utter a piercing shriek, or a cry of triumph, and this would be the signal for a general hysterical outbreak, accompanied by many remarkable physical symptoms. Of these the most common were falling in convulsive spasms, jerking, dancing,289 barking like dogs, fainting, crying, singing, praying and cursing. Sometimes whole companies were seized with uncontrollable laughing fits, called the holy laugh. At a meeting in East Tennessee, six hundred began jerking at one time. In many instances sensibility would be lost and the extremities would be cold, while the face was flushed. In some places the sufferers were laid out in rows and squares in the churchyard until they should recover. From a medical point of view we should call this epidemic chorea, but its more exact physiology I have already referred to. When closely examined, the phenomena lose a part at least of their mysterious character. We must remember that religious emotions are powerful, deep and ancient. The effect, furthermore, is increased by the general epidemic excitement, by the element of large and unwonted gatherings of people, by imitation, by the stimulating music and by the fearfully exciting power of human shrieks and wild cries and prayers. Such a nervous condition induced in an individual must have two results: first, the escape of the unusual nervous excitement in motor channels, giving rise to the choreic movements; and second, the paralysis of the higher brain centers, resulting in various hypnotic phenomena and reversionary morality and mentality.
Many of these scenes were repeated in the great revival that swept New York and the Middle States, beginning in the year 1832. In these meetings preachers who kept cool and reasoned logically were not listened to. There was rather a demand for the wild, impetuous, vociferous, physically impassioned oratory of the rude man. As an example of reversionary morals in this epidemic, we may notice the fact mentioned by Albert Rhodes that in response to visions many men put away their own wives and took others from their neighbors.
From the psychological point of view perhaps the most instructive of all epidemics is the demonophobia or witchcraft mania which raged from the end of the fifteenth to the end of the seventeenth centuries. The savage’s fear of demons and of unseen supernatural agencies lurking in every forest and moor now took hold of the modern world and turned the people, not into brutes and devils as we figuratively say, but simply into the original savages from which they came, whose basal instincts they still carried in their lower nervous centers, to be brought out under the influence of a social craze. The ecclesiastical authorities, both Roman and Protestant, led in this homicidal frenzy, while sedate judges, learned jurors and wise legislators lent their zealous aid. It spread in true epidemic form all over the Continent and into England and Scotland, even to America. Distinguished jurists declared that ordinary methods of trial should not be used for this offence, for so difficult is it to bring proof of the crime of witchcraft, that out of a million of witches not one could be convicted if the usual course of290 justice were followed. One contemporary of undoubted authority wrote that he saw a list of three thousand witches that had been put to death during the time of the Long Parliament alone. In this reign of demonophobia the psychological phenomena of the craze are well illustrated. The exciting cause was a widespread contagious and epidemic fear. The result was a recrudescence of the barbaric instincts of cruelty, torture and homicide, accompanied by a loss not merely of reasoning power, but apparently of common sense, so that intelligent men seemed to believe that old women blasted the crops in the fields and the offspring of animals, and raised storms and whirlwinds. The cruelty characteristic of the savage is again noticed in this case. In the witchcraft persecutions, the victims were commonly weak women, particularly the more helpless old and young, while the character of the inflictions was such as is peculiar to primitive people, viz., torture and burning alive. The perfidy of the savage is also noticed, as in innumerable instances the victims were led to believe that they would be spared if they made a confession, and were then put to death. To elude a legal requirement that torture should not be repeated, the most horrible tortures were ‘continued’ from day to day.
The psychology of crazes is clearly seen in certain of its aspects in the homicidal manias that have swept over communities or whole countries at frequent intervals in the world’s history. The homicidal impulse itself is one of the most primitive and basal of all impulses. The reason for this is apparent. The history of man has been a history of warfare and of struggle for existence. It has been man against man, tribe against tribe, nation against nation. Habits like these are not quickly unlearned, and reversion to them in times of social disturbance is not strange. In the massacre of St. Bartholomew we have a typical instance of the homicidal mania. The necessary conditions were, first, great emotional excitement caused by religious fanaticism acting as an inhibitory agent upon the higher brain centers and allowing the primitive impulses to act unchecked; second, the removal of external and customary restraints, effected in this case by the royal decree; and third, the mental effects of imitation and suggestion. These conditions being all supplied, the French people resolved themselves speedily into assassins and cut-throats, and enjoyed a homicidal debauch. Begun in Paris, the massacre spread in true epidemic form throughout France, until fifteen or twenty thousand people had perished.
These homicidal manias have, of course, been very frequent in history. The decivilizing influence of the craze is, however, most perfectly illustrated in the various scenes of the French Revolution. Here the overturning of the social and religious order itself acted in part as the unsettling and emotionally exciting cause. The usual results followed.291 The effect of social excitement in paralyzing the intellect was shown in this case in the wholesale and useless destruction of women and children. Furthermore, this reversion to the manners of the savage carries with it its appropriate mood. The slaughterers are not like demons, as we imagine demons to be, but rather like thoughtless children. There is merriment and much gayety, and there is dancing and singing around the corpses, and seats are arranged for the ladies, who are eager to enjoy the spectacle; and finally the victims are made to pass through a double row of executioners, who carve them into pieces gradually, so that all can saturate themselves with the sight of the bloodshed.
Although in some cases wars may be coolly planned by the people’s leaders for personal or political reasons or for purposes of national conquest, still they all depend for their successful issue upon the homicidal impulse in the masses of people. This is called the war spirit and is always of an epidemic character. It may have any degree of ferocity or mildness. It has a tendency to be periodic, so that if it has slumbered for a considerable period a very slight cause is sufficient to awaken it. A mere boundary line in Venezuela, in which this country had but a remote interest, was sufficient a few years ago to excite this war spirit in a milder form, when a curious craze for a war with Great Britain flowed like a wave across this country.
Any war will furnish instructive material to the student of social psychology. In the late Spanish-American war, for instance, we all felt the war spirit which flowed in epidemic form across the country and engulfed it. The first motive of the war, the altruistic desire to free an oppressed people, was of the ideal glittering kind, well fitted to excite the emotions of the masses. A dramatic event further fans this emotional flame, and at once the aggregate personality of the nation is in a condition of automatism, where primitive instincts, such as revenge and lust for the paraphernalia of war, are no longer checked by the more lately acquired moral principles. Congressmen, editors, members of peace societies, ministers of the gospel, forget their long and patient efforts to establish means for settling national differences by arbitration and join lustily in the war cry, and the psychologically curious spectacle is presented of a great nation, priding itself as a leader in the world’s morals, giving to the appeal of a weaker nation for the arbitration of a dispute the answer of shot and shell. Although the motive of blood for blood is a moral motive belonging to a bygone age and in individual ethics has long been outgrown, yet collectively, under the influence of the war craze, we revert to it, and it is shamelessly proclaimed from platform and editorial room and vigorously applauded by the people. We have seen that cruelty and the persecution of the weak by the strong were among the reversionary symptoms of the social292 epidemic in many instances. We may notice curiously enough a trace of these qualities here, where the fact that our enemy was a greatly inferior power does not detract in our eyes from the brilliancy of our victories, though in the ethics of the individual such a circumstance would put us to shame. In all this we proceed strictly in accordance with international law, but international law itself is only international custom and is the mere expression of the wonted behavior of the aggregate personality, particularly in times of war. As such it does not represent the highest ethical development of man, but that lower stage of development to which he reverts in times of social excitement. From this point of view it is possible to understand why international ethics is so far behind individual ethics. Personal disputes were once settled by brute force as international disputes are now settled. There is no reason to doubt that the latter will, somewhat later in the history of civilization, be settled by courts of arbitration and enforced by a system of police as the former now are.
The considerations now before us show the futility of peace congresses in that part of their work which contemplates the enforced substitution of arbitration for war. Peace congresses are not social movements. They spring from the efforts of individual men, leaders in social reform. They belong to the upward ethical movements led by individuals, the slow, painful climbing towards higher moral and intellectual standards. These congresses may meet and discuss arbitration and perfect an international program, but they labor in vain, for they forget that social man has a double personality and that the personality that meets and deliberates in the peace congress is not the personality that, under the influence of the war craze, thrills with emotion and acts from ancient and deep rooted impulses and motives. When the war spirit sweeps over a country the social personality passes into a condition not unlike that of hypnosis and is ruled by a different set of moral principles. It should not be understood from this that peace congresses are useless. They are a part of an educative system whose influence in the end will be strong enough to react upon the secondary social personality and determine its behavior.
Among crazes of a different kind, we may notice financial crazes as an interesting type, falling under the same laws as those mentioned. Both in panics and in speculative manias we observe again a species of hypnotization. In the case of the latter the ordinary business shrewdness which characterizes the dealings of the individual in a normal state and which depends upon the activity of late developed association tracts in the brain, is to a large extent lost. The memory is impaired and what in general we may call prudence is lacking.
The psychology of the speculative mania is very simple. There is first, greed, furnishing the necessary emotional excitement; then imitation;293 then precipitate, unreasoning action. In the panic, the psychological sequence is the same, except that fear takes the place of greed. The stampede among animals may be taken as the type of all panics. It is a reflex phenomenon consisting merely of contagious fear and precipitate, unintelligent flight. Fear and flight constitute a most primitive form of mental action, equalled in primitive character only by that other form whose survival we have seen illustrated in wars and homicidal manias, viz., anger and combat. Although the individual has long outgrown these simple reflexes, yet in social excitement he reverts to them. The recrudescence of the first of these two forms is seen in the case of panics in theatres and burning buildings, where social fear is followed by unintelligent flight, there being a temporary paralysis of reason, prudence, the power of choosing means to ends, respect for women and consideration for the weak and feeble.
The limits of this paper permit me only to refer to other forms of the craze illustrating the same laws. In fads and fashions of all kinds, the behavior of the social personality is different only in degree from that already described in the more serious epidemics. The law of imitation is the same, but there is less excitement and emotional disturbance and consequently a lesser paralysis of the higher mental faculties and a lesser return to barbaric impulses. Whereas the others may be called forms of social paranoia, these may be called forms of social monomania. A single idea fills the public mind, and as a result this idea is unduly exalted as to its importance and worth. The higher mental powers are paralyzed only so far as that there is a perverted judgment as to the relative importance of things and consequently a more or less distorted view of the world and its values. Perhaps the simplest form of this craze is seen in the epidemic character of children’s games. At different times of the season different games completely fill the social consciousness of the child-world, so that for the moment there is no interest in any other game. New and interesting sports, such, for instance, as golf, often fill the social adult consciousness in the same way. Then there are social and literary fads, crazes in musical airs, fashions in dress, furniture, houses and carriages, without number. Crazes of all kinds have found a prolific soil in America. The American mind is highly suggestible. One fad after another rages over the country and in some cases reduces the aggregate mind to a condition of idiocy. The Dewey craze in New York City last year is an illustration of this. Nothing but a sort of hypnotic distortion of intellectual vision could cause grown men to stand in line for an hour in order that they might sit for an instant in the chair in which the hero sat during the review, or to fight for shreds of the flags and awnings that decorated the platform.
Sporadic social reform movements take the form of crazes and illustrate294 the same laws. One recalls the Woman’s Crusade in 1873, the result not of a rational plan but of imitation, and the Granger movement and the Farmers’ Alliance and the greenback craze and the silver craze and many others.
Since Aristotle we have been told that man is a social animal and that to study him as he really is we must not isolate him from society. The evident truth of this may lead us to forget that it is but a half truth and the uncritical acceptance of it will lead us wholly astray in our sociological study. The inference which we seem compelled to draw from studies in social psychology is that social man is, in his ethical and intellectual development, many stages behind the individual man. The progress of civilization is a slow, painful, upward climbing, in which individuals are the thinkers, the planners, the promoters and the leaders. The mind of society, on the other hand, using the phrase in the sense defined, is an imitative, unreflective, half-hypnotic, half-barbaric mind, always acting as a drag upon the upward and forward movement, and, in times of crazes, epidemics and social cataclysms, gaining temporary dominance and causing disastrous relapses to a lower plane of civilization.
In the following pages an effort is made to apply some of the results of recent chemical research to the earlier history of the earth. It is hoped that the main facts brought out may be readily grasped by those who have never studied chemistry, and that each link in the chain of events will be made evident to those who have mastered the rudiments of this science.
Chemical action involves change of composition. Substances more or less complex may be broken down into simpler substances, or from several simpler substances a complex substance may be built up. From the complex ore of copper found in nature the simple element copper is obtained. From the elements sulphur and oxygen and the simple substance water the complex sulphuric acid is built up. Within the last few years the high temperature of the electric arc—the heat generated by a powerful electric current playing between two carbon poles—has been employed in bringing about chemical changes which do not occur at ordinary temperatures nor at those obtainable by burning fuel. The electric furnace is used industrially to make calcium carbide from lime and coke, carbon silicide (carborundum) from coke and sand, and the metal aluminum from its compounds.
Chemical changes at high temperatures have long been an object of research, but it was not until the introduction of the electric furnace that it was possible to command temperatures high enough to make exhaustive studies. In the last few years several chemists, especially Moissan, of Paris, and his pupils, have done systematic work with the aid of the arc furnace. The furnace used in the laboratory for high temperature work is a small and simple apparatus; Moissan’s furnace is a block of quick lime a little longer and wider than a page of this magazine and about three inches thick. A rectangular cavity is cut on the upper surface of this block. A similar block forms the cover. In opposite grooves between the top and bottom piece are placed the carbons, such as are used in ordinary arc lights. The arc plays across the cavity in such a manner that the substance to be heated is not brought into the arc itself, which is vaporized carbon, but below it. The cavity thus represents a tiny reverberatory furnace; the arc heats the roof and sides to an intense heat, which is radiated on the open dish296 or closed crucible or tube containing the substance heated. This is the simplest form of laboratory furnace. Various modifications are used, but in all the size is small and the arrangement simple. A powerful arc plays in the smallest possible cavity with the object of attaining the maximum of temperature, expense and duration of material being secondary considerations. Lime and magnesia are the best materials, because they are at the same time the most refractory substances available and are poor conductors of heat. A furnace top one and one half inches thick may be heated by so powerful an arc that the melted quick lime drips from the inner surface, while the outer surface is scarcely warm to the touch of the hand. Moissan has utilized in these little furnaces currents of electricity of varied strength, the lowest being that given by a four horse-power dynamo, the highest that generated by three hundred horse power. The highest temperatures obtained were about 3,500° centigrade (6,300° Fahrenheit), with the heat constantly increasing; the limit to the obtainable temperature—as far as the experimental evidence showed—was merely the lack of any known substance refractory enough to bear the heat; for at the temperature mentioned quick lime and magnesia not only melt but are changed into gases, so that the furnace was filled with the vapors of its own material.
The effect of the heat on single substances is very interesting. Refractory metals, such as iron, manganese, uranium, platinum, melt rapidly and then become gaseous; the most refractory non-metallic elements, silicon, boron, carbon, are also changed into the gaseous form. Very refractory compounds are broken down into simpler ones. Magnesium pyrophosphate yields phosphorus, magnesium oxide and oxygen. Asbestos—a magnesium silicate—gives as chief product magnesium silicide; the other substances formed being silicon, silicon dioxide and a little magnesium oxide.
Such are the astounding changes wrought by simple heat upon those substances which we are accustomed to regard as infusible. It must be remembered that the range of temperature which chemists employ in ordinary laboratory work is not very great and that the conditions of work in the laboratory and of nature’s work on the earth’s surface at the present day favor the formation of two classes of compounds—the oxides and their hydrates. Although air is a mixture consisting mainly of four parts of nitrogen and one of oxygen, atmospheric nitrogen is generally inert at ordinary temperatures, and it is the oxygen of the air which is the more important factor in the growth of living things and in changes in lifeless matter. Water, a compound of oxygen and hydrogen, is present everywhere, either in the liquid form or as vapor in the air; even in the flame of the hottest fires there is water vapor in abundance, since water is one of the chief products of combustion of most forms of fuel. Is it a297 wonder that under such conditions we find the earth’s crust to contain the elements chiefly compounded with oxygen? Was this always so? Are we justified in supposing that conditions may have prevailed—nay, must have prevailed—in former times on the earth’s surface, which gave to other elements as important or more important functions than to oxygen? The answer to these questions must be sought in the results of the chemistry of high temperatures.
First let us consider the conditions of existence of the omnipresent water. Water begins to break down into its components, hydrogen and oxygen, at 934° centigrade; at 2,500° centigrade (4,500° Fahrenheit) the decomposition is complete. In other words, water vapor cannot exist at temperatures above 2,500°, but the hydrogen and oxygen exist in the free state.
Astronomers tell us that refractory elements like iron, silicon and carbon, perhaps disassociated into still simpler substances, are present as vapor in the atmosphere of the sun and that many others of our well-known elements, including hydrogen, are also present in this glowing atmosphere, while the heat of the sun’s surface and that of the hotter stars is vastly higher than that of the electric furnace. Geologists believe that the evidence at their disposal points to a similar period of great heat in the early history of the earth. It may be considered, then, that temperatures higher than those of the electric furnace prevailed in former times on the earth’s surface.
Let us now return to the study of the results obtained with the electric furnace. The following reactions are especially important. If metals, or refractory non-metals, or metallic or non-metallic oxides, or complex silicates, are heated to the higher temperatures in contact with carbon, boron, silicon or compounds of these three elements with oxygen, the result generally is that very refractory carbides, borides or silicides of the metals or non-metals are formed. In other words, those complex substances which form the chief constituents of the outer crust of the earth at the present day are decomposed at high temperatures, and simple compounds of two elements—so-called binary compounds—are formed. Four classes of these binary substances seem to be especially stable at high heat—the carbides, borides, silicides and oxides; but the oxygen of the metallic oxides tends to pass off as an oxide of carbon, if carbon be present.
At somewhat lower temperatures nitrogen is very active and the nitrides of many metals are readily formed. An excellent example is shown by heating a mixture of carbon and of an oxide of titanium (titanic acid). When heated by a feeble current the acid is simply reduced, forming a lower oxide of titanium; with a more powerful current the mass is completely changed into the nitride of titanium, the nitrogen coming from the air; with a very powerful current this is298 changed into pure carbide, as the nitride cannot exist at the higher temperature, and the nitrogen escapes, carbon taking its place. At still higher temperatures hydrogen acts on many metals, forming hydrides. The carbides and other compounds of some metals are not stable at high temperatures, being reduced by gaseous carbon to the free metals, which remain then in the gaseous form.
At that period of the earth’s history when the temperature was as high as that easily obtained in the electric furnace, we have the sanction of geologists for picturing the earth’s surface as an ocean of molten matter surrounded by a glowing atmosphere. This molten surface must have consisted of binary compounds such as those mentioned above, and probably contained some refractory elements, metals and non-metals, in the free state. The atmosphere contained free hydrogen, oxygen and nitrogen, gaseous binary compounds like the oxides of carbon, metals in the gaseous form and many non-metallic elements like sulphur and chlorine. In the atmospheric region furthest removed from the molten surface violent chemical reactions occurred between the heated elements, forming compounds which were again dissipated into their elements by the heat given off in the act of formation or radiated from the glowing surface below.
Under the enormous pressure of this atmosphere the liquid surface of the earth solidified at very high temperature. Whether the earth’s mass solidified from the centre outward or by forming a solid crust over a liquid interior, is a question to be decided by physicists and geologists. We will consider only the outer crust and the atmosphere. As the surface and the atmosphere above it gradually cooled, the formation of nitrides, and later of hydrides, sulphides and chlorides, occurred.
The conditions now attained may have been fairly stable as long as the temperature of the surface and lower regions of the atmosphere were high enough to prevent the union of the atmospheric oxygen and hydrogen, or to decompose the water forming in the outer regions of the atmosphere. As soon, however, as by further cooling, water came into contact with the earth’s surface, very violent reactions occurred, which were supplemented by other equally violent reactions when the cooling process permitted the formation of the ordinary mineral acids.
The reactions of water and of acids on many of the binary compounds are so important in determining the present composition of the earth’s crust that they must be considered in detail. The carbides, nitrides, chlorides, sulphides and hydrides of most elements, and some silicides, are decomposed by water, or else by dilute acids, forming the hydrogen compounds of carbon, nitrogen, chlorine, sulphur and silicon respectively, and the oxide or hydroxide of the other element. Thus calcium carbide and water give calcium hydroxide and acetylene, a299 hydro-carbon. Aluminum carbide yields alumina and methane (marsh gas), another hydro-carbon, the chief constituent of ‘natural gas.’ Other carbides yield crude petroleum. The nitrides yield ammonia, which is the hydrogen composed of nitrogen. The chlorides give hydrochloric acid, the sulphides sulphuretted hydrogen and the silicides the hydrogen silicide. The metallic hydrides yield free hydrogen.
The violence and the magnitude of some of these reactions almost baffle the imagination. Let the reader drop a piece of calcium carbide as large as a small marble into a little water in a cup; there is a rapid action, a gas (acetylene) is given off, which burns with a smoky flame if a lighted match is held over the cup. (The experiment should be tried in the open air.) So much heat is generated in the reaction that the cup becomes hot. Nearly four per cent. of the earth’s outer crust is calcium; all this was at this period of the earth’s history in the form of carbide. Imagine all the vast limestone mountain ranges of the present day as carbide, and try to realize the effect when water fell on any considerable area. The heat generated would be so enormous that in a moment the acetylene would ignite and burn, forming oxides of carbon and water vapor, which would in turn decompose, throwing the jets of glowing hydrogen and oxygen vast distances into the atmosphere, there to cool and reunite to water. The decomposition of other carbides, of the hydrides and silicides, as well as the formation of hydroxides by the action of the lighter metals on water, would produce similar phenomena, as the substances formed are combustible gases, or liquids or solids easily volatilized. This is no wild fantasy, but a conservative statement. Similar reactions are taking place at the present day in those stars whose cooling process has advanced far enough; a case in point is that of the so-called ‘temporary stars.’
Extremely violent reactions are taking place constantly in the atmosphere of the sun. The sun’s chromosphere, or outer layer of its atmosphere, consists mainly of hydrogen, and jets of glowing hydrogen are thrown to great heights above the chromosphere; these jets or ‘prominences’ have been frequently observed to have a height of 100,000 miles, and prominences of more than double this height are reported by observers. The most conservative estimates assume temperatures of the sun’s surface so enormous that that of the electric furnace is insignificant in comparison, and we can have no conception of the chemical changes occurring under such conditions. Whether one believes, with Lockyer, that the chemical ‘elements’ are disassociated by the sun’s heat into simpler substances or not, it is clear that very violent chemical reactions are in progress, and if we realize that the known chemical reactions increase in intensity with increase in temperature, it does not seem strange that at the sun’s temperature the reactions occurring should cause disturbances like those observed.
300 Returning to the earth, let us consider the products of these violent reactions. The hydrogen and hydrides of boron, silicon, sulphur and carbon, combined with the oxygen of the atmosphere, forming water and boric, silicic, sulphurous and carbonic acids, which in turn acted on the metallic oxides and hydroxides, forming sulphites, carbonates, borates and simple and complex silicates; some quickly, some slowly, some at low temperatures and atmospheric pressure, others at high temperatures in liquid or semi-liquid condition and under the pressure of rock masses above. To determine the relative age of existing rock layers, or the mode of their formation, whether by eruptive action, by surface heat, by deposition of finely divided material under water, or by metamorphic changes of the cooled silicate under subsequent action of water, pressure and heat, is the province of the geologist. The present writer refrains from an opinion whether any of the first formed solid crust could or could not survive to the present day in its primary form, considering the exposure to water, acids, heat and pressure which it suffered.
Yet an idea may be formed of the condition of the earth’s surface when it had cooled so far that the more violent chemical action had ceased. It consisted chiefly of silicates, simple and complex; of some of the original binary compounds, which are scarce affected by water or acids, such as the silicide of carbon (carborundum), of stable oxides, chlorides and sulphides, with other compounds in smaller proportion, and free elements in greater proportion than at the present day. Everywhere, from crevices in the surface, hydrocarbons, phosphoretted hydrogen (phosphine) and ammonia were issuing as gases; the atmosphere was heavy with these gases and with carbon dioxide.
No scientific observations thus far show how or from what definite compounds plant life or animal life was first evolved from lifeless matter; but it is certain that the materials were much more abundant and the conditions more favorable at the period when it was evolved than at the present day. An ocean much warmer and less saline than now, a damp atmosphere like that of a hothouse, an abundance of plant food and a choice of raw material, were at hand. The chief foods required for plant life are nitrogen in the form of ammonia or nitrates, carbon dioxide, phosphorus as phosphates, sulphates of lime, of magnesia and of the alkalies, and water. As to the raw material for the first formation of the living cell, it is impossible to say what compounds of carbon were employed; suffice it to note that the known simple and complex binary compounds of carbon were there ready for use; the hydrocarbons, carbon monoxide and carbon dioxide were oozing from the earth’s surface, from the ocean floor as well as from the land, or hanging heavy in the air above it. If warmth or increased pressure were desiderata, an ocean warm to its greatest depths could afford any pressure301 required. From the decomposition of the nitrides and phosphides below the surface, ammonia and phosphine were escaping into the ocean and into the air. The conditions then during long periods of time were especially favorable for marine life, and as sand and mud accumulated on the rocky surface of the earth, for land plants; the absence of a thick soil being more than compensated for by the abundance of plant food, notably of carbon dioxide and ammonia.
The statement may be found in excellent modern text-books of chemistry that ammonia is always formed by the decomposition of plants and animals, accompanied by the further statement that ammonia is a requisite for plant food. No plants—no ammonia; no ammonia—no plants. If this were true, the beginning of plant life would indeed have been a struggle for existence; that it is not true is shown above. This decomposition of nitrides has ceased practically on the actual surface of the earth at the present day because the nitrides have all been decomposed; yet it may be mentioned that specimens of rock freshly quarried in Sweden were recently found to give off ammonia when wet with water, showing the presence of nitrides. Below the actual earth’s surface it is probable that nitrides still exist in large quantity, for ammonia is one of the constituents of volcanic gases; to believe that volcanic ammonia is a product of plant or animal decomposition is difficult; to suppose it formed by the action of steam on nitrides in the earth’s interior is simple.
Much the same may be said of the presence of carbides. While they no longer exist on the surface, there is no doubt of their existence in the interior of the earth, and the volcanic gases contain their decomposition products. In this connection the theory—first put forward by Mendelèeff and since supported by Moissan—of the origin of petroleum, may be mentioned. These writers favor the hypothesis that it was formed by the decomposition of carbides by water under pressure; and while the evidence at hand perhaps favors the belief that the petroleum of the more important oil fields owes its origin to decomposition of the lower forms of marine animal life, yet there can be no doubt that petroleum may be formed by carbide decomposition, and it seems probable that natural gas is in part at least a result of the same action.
Partly in deference to the report of the United States Weather Bureau, from which it appeared that the chance of a fair eastern sky on the morning of the eclipse was about 8 to 1, and after examination by Mr. Abbot of many stations in North Carolina, Wadesboro, of that State, was selected early in April as the site of the Smithsonian observations. The advantages of Wadesboro being also recognized by Professor Young, of Princeton, Professor Hale, of Yerkes Observatory, and the Rev. J. M. Bacon, of the British Astronomical Association, it came about that four large observing parties, besides several smaller ones and numerous excursionists from the surrounding country, were all joined to produce at Wadesboro one of the largest company of eclipse observers ever assembled for scientific purposes. It is a matter for congratulation that the sky at Wadesboro upon the day of the eclipse was cloudless and clearer than the average, so that the efforts of the observing forces were not thwarted by any circumstances beyond their control. The provisions of the Mayor and authorities of Wadesboro for preventing intrusion before and during the eclipse, and thus securing an undisturbed field of operations, deserve especial recognition. Further than this, the many acts of courtesy and hospitality to the visiting astronomers on the part of the townspeople will long be remembered by the recipients.
The Smithsonian party proper consisted of thirteen observers, and included Mr. Langley, Mr. Abbot, aid acting in charge of the Smithsonian astrophysical observatory; Mr. Smillie, in charge of photography; Mr. Putnam, of the United States Coast Survey; Mr. Fowle, Mr. Mendenhall, Mr. Child, Mr. Draper, Mr. Gill, Mr. Kramer and Mr. Smith. Included with these, the Rev. Father Searle and the Rev. Father Woodman gave most valuable assistance. Mr. Hoxie, of Port Royal, S. C., and Mr. Little, of Wadesboro, rendered valued assistance to Mr. Putnam during totality.
Professor Hale, of the Yerkes Observatory, was a member of the party, while still in general charge of the Yerkes expedition, and his counsel and aid were of the greatest service. Mr. Clayton, of Blue303 Hill Meteorological Station, occupied a part of the grounds of the Smithsonian party.
The main object of investigation was, of course, the corona, and of this, (first) a photographic and visual study of its structure; with, (second) a determination by the bolometer whether appreciable heat reaches us from it, and, if possible, an examination of the form of its spectrum energy curve.
The writer had been particularly struck, when observing the eclipse of 1878 on Pike’s Peak, by the remarkable definiteness of filamentary structure close to the sun’s limb, and had never found in any photographs, not even in the excellent ones of Campbell taken at the Indian eclipse of 1898, anything approaching what he saw in the few seconds which he was able to devote to visual observations at the height of fourteen thousand feet. His wish to examine this inner coronal region with a more powerful photographic telescope than any heretofore used upon it, was gratified by the most valued loan by Prof. E. C. Pickering of the new 12-inch achromatic lens of 135 feet focus, just obtained for the Harvard College Observatory. This lens, furnishing a focal image of more than 15 inches diameter, was mounted so as to give a horizontal beam from a cœlostat clock-driven mirror by Brashear, of 18 inches aperture, and used with 30-inch square plates. To supplement this great instrument, a 5-inch lens of 38-feet focus, loaned by Professor Young, was pointed directly at the sun. This formed images upon 11 × 14 plates moved in the focus of the lens by a water clock. Specially equatorially mounted lenses of 6, 4 and 3-inch aperture, driven by clock work, were provided for the study of the outer corona, and the search for possible intra-mercurial planets.
For the bolometric work, the massive siderostat with its 17-inch mirror, and a large part of the delicate adjuncts employed at the Smithsonian Institution in recent years, to investigate the sun’s spectrum, was transported to Wadesboro. The excessively sensitive galvanometer reached camp without injury even to its suspending fibre, a thread of quartz crystal 1-15,000 inch in diameter.
Besides these two chief aims (the photography and bolometry of the inner corona), several other pieces of work were undertaken, including the automatic reproduction of the ‘flash spectrum’ by means of an objective prism with the 135-foot lens, the photographic study of the outer coronal region, including provision for recognizing possible intra-mercurial planets, already alluded to, visual and photographic observations of times of contact, and sketches of the corona, both from telescopic and naked-eye observations.
The assignment of the observers was as follows: Mr. Langley, in general charge of the expedition, observed with the same 5-inch telescope used by him on Pike’s Peak in 1878, which was most kindly lent304 for this special comparison by Professor Brown, of the United States Naval Observatory; C. G. Abbot, aid acting in immediate charge, assigned with C. E. Mendenhall to the bolometer; T. W. Smillie, having general direction of the photographic work, made exposures at the 135-foot telescope; F. E. Fowle, Jr., assigned to the 38-foot telescope; Father Searle, directing the assembled telescopes for the outer coronal region, and for intra-mercurial planets, assisted by P. A. Draper and C. W. B. Smith, exposed two cameras of 3-inch aperture and 11 feet focus, and two of 4½-inch aperture and 3½ feet focus, all four of these telescopes being mounted on a single polar axis driven by an excellent clock; De Lancey Gill, assisting Mr. Smillie, removed the flash spectrum objective prism at second contact, and made a single long exposure with a 6-inch photographic lens of 7½ feet focus equatorially mounted; Assistant G. R. Putnam, who, by the kindness of the superintendent of the United States Coast Survey, was detailed for latitude,C longitudeD and time observations, also observed contacts, directed the striking of signals by Mr. Little, and rendered other valuable services. Mr. Putnam was assisted in recording contacts by Mr. Hoxie. R. C. Child, observing with a 6-inch telescope of 7½ feet focus, made sketches with special reference to inner coronal detail, and was in addition charged with all electrical circuits for chronograph and automatic photographic apparatus. Father Woodman, with 3½-inch telescope, observed contacts and made sketches.
The first detachment, consisting of Messrs. Abbot, Fowle, Kramer (instrument maker) and Smith (carpenter), reached Wadesboro May 4th, and were soon joined by Messrs. Draper and Putnam. The latter returned to Washington after a short but satisfactory latitude and longitude campaign, reaching Wadesboro again just before the eclipse. Other members of the party reached camp on and after the middle of the month. The first comers found a very satisfactory shed already erected and piers begun. Not a day passed from the time of the arrival of the apparatus, May 7th, to the day before the eclipse, that was not fully occupied in perfecting the arrangements.
The most striking portion of the installation was the line beginning at the northwest pier, with its equatorial and cœlostat, continued from thence south of east by the two great diverging tubes of the 135-foot telescope and spectroscope. These tubes were covered with white canvas, presenting the appearance of two immensely prolonged ‘A’ tents, ending beyond the photographic house, where the 38-foot telescope tube pointed east and upward at an angle of 42° with the horizon. When the equatorial, with its large special conical tube camera, with all this long-branching extent of white canvas ending in the uplifted tube of305 the 38-foot telescope, was seen in the light of the moon, the extensive field with these preparations, exhibited a still more picturesque scene than by day.
Less imposing, and perhaps more ungainly was the combination of four great cameras under the main shed, designed to search for new planets and to depict the outer corona. These might well be described as like a cabin and outbuilding, mounted on a polar axis, yet, despite their awkward proportions, they were made to follow very accurately.
The morning of the eclipse dawned cloudless and very fairly clear. Deep blue sky, such as the writer had seen on Pike’s Peak, of course, is not among the ordinary possibilities of an eclipse, but the milkiness of the blue was less pronounced than is usual in the summer season, and all felt that the seeing promised well.
At fifteen minutes before totality a series of rapid strokes on the bell called every one to his post, and one minute before the expected contact five strokes were given as a final warning. Coincidentally with the actual observation of the second contact by Mr. Putnam, the first two strokes upon the bell sounded, and the work began. After 82 seconds (the duration of totality from the Nautical Almanac was 92 seconds), three strokes were given as a signal to stop the long photographic exposures. Scarcely more than five seconds after this the sun’s crescent reappeared. The duration of totality, as observed by Mr. Putnam, was approximately 88 seconds.
To visual observers the sky was notably not a dark one. No second magnitude stars were observed with the naked eye, and most of the on-lookers saw only Mercury conspicuously, though Venus was distinguished at a low altitude and Capella also was seen. So high a degree of sky illumination can not but have operated unfavorably in the study of the outer corona or in the search for intra-mercurial planets, and this is to be remembered in connection with what follows.
A deepened color in the sky, a fall of temperature and a rising breeze were distinctly noticeable. No change in direction of the wind was noted. Shadow bands were seen, but those who attempted to measure their velocity found them too rapid and flickering for any great exactness in this determination. There was tolerable unanimity among independent observers as to their size and distance apart (about five inches), though some thought this less as totality approached.
It was noticed that the birds grew silent just before and during totality, but true to their nature, the English sparrows were last to be still and first to begin their discussion of the eclipse, after the return of light.
The attention of all visual observers was at once caught by the306 equatorial streamers. Father Woodman’s comparison of the appearance of a structure of mother of pearl was generally recognized as good, but different observers differed on the color estimate. A yellowish green tinge was noted by the artist of the party, Mr. Child, while to others the light was straw colored or golden.
The general coronal form to the naked eye was nearly that of the small annexed photograph, which, though taken by one of the smaller objectives, gives a good view of the relative intensities. The same extensions of the equatorial corona could be followed by the naked eye from 3 to 3½ solar diameters.
The visual telescopic observations of the writer gave little indication of the finely divided structure of the inner corona which he had noticed at Pike’s Peak. Structure, to be sure, was evident, but not in such minute subdivision as had then been seen, and though one remarkable prominence as well as several smaller ones was visible, the coronal streamers did not give to the writer the impression of being307 connected with these prominences, though the relationship of some of them to the solar poles was abundantly manifest.
Comparing notes after totality, all observers reported a successful carrying out of the programme. The greatest interest centers in the direct coronal negatives taken with the 135-foot telescope. Mr. Smillie exposed six 30 × 30 plates during totality, with times ranging from one half a second to sixteen seconds, and three others were exposed by him immediately after the third contact.
At this writing only a part of the negatives taken have been developed. Their general quality may be inferred from the examples here given, after due allowance for the great loss suffered by translation onto paper even with the best care.

Fig. 1 is a view taken with one of the smaller objectives (6 inches), given here to afford the reader an idea of the general disposition of the coronal light. The upper part is the vertex in the inverted field.
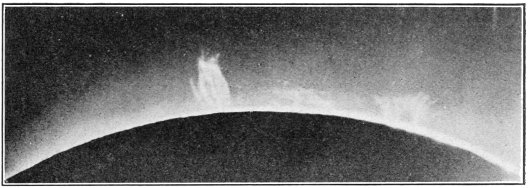
Fig. 2 is a portion of one of the great 15-inch circular images obtained with the 135-foot focus telescope. It was obtained in the great disc in the last exposure during totality of 8 seconds, showing one of the principal prominences then on the sun’s disc, with the disposition of the lower filaments near it.

Fig. 3 is a portion of one of the same set of plates, but taken with a 16-second exposure. The part near the sun has, of course, been intentionally over-exposed, in order to better exhibit the remarkable polar streamers, extending here to a distance of about six minutes from the sun, but seen still further in Mr. Child’s telescopic drawing (not given.)

Fig. 4 is a view of a small part of the great apparatus on the field, including the terminus of the horizontal tube with its canvas covering, which has been described as like an extended ‘A’ tent. The photographic room is seen at the end of the tube, and beyond that the tube308 containing the lens loaned by Professor Young pointing directly skyward.
That it will be impracticable to give here all of the disc of the moon in the large photographs, will be evident when it is considered that the lunar circumference on each plate is about 4 feet; but it will be inferred from the examples that the prominences and polar streamers as well as their features, appear in imposing magnitude and detail.
Many of what it is hoped will be the most interesting photographs still await development, but Mr. Smillie’s thorough preparation is promising adequate results.
Mr. Abbot, with aid of Mr. Mendenhall, appears to have measured the heat of the corona, and in spite of previous efforts, this is probably the first time that it has been really shown to exist. For five minutes before second contact, the bolometer was successfully exposed to the region of the sky close to the narrowing crescent of the sun where the corona was shortly to appear. A diaphragm was interposed in the beam having an aperture of only 0.4 sq. cm. Deflections, rapidly diminishing from 80 to 6 mm. were obtained, the last being about 40 seconds before totality. Then the diaphragm was opened to 290 sq. cm. and a negative deflection of 13 mm. was observed after totality, where these positive deflections had just been found, showing that the corona was actually cooler than the background which had been used at the room temperature. Next the black surface of the309 moon was allowed to radiate upon the bolometer, and the still larger negative deflection of 18 mm. was observed.
The important result was that the corona gave a positive indication of heat as compared with the moon.
This heat, though certain, was, however, too slight to be sub-divided by the dispersion of the prism with the means at hand.
The negatives taken to depict the outer corona show from three to four solar diameters extension for the longest streamers. The equatorial ‘wings,’ as they recede from the sun, are finally lost in an illuminated sky, without any indication of having actually come to an end.
No attempt to carefully examine the plates taken for intra-mercurial planets has yet been possible. It is, however, as has been remarked, doubtful if the very faintest objects will be found, in consideration of the considerable sky illumination during totality. However, Pleione in the Pleiades (a star of the 6.3 magnitude), is plainly seen on one of the plates, and some smaller ones are discernible.
On the whole, the expedition may be considered as promising to be very satisfactory in its results, and that it was so is largely owing not only to the efficient care of Mr. Abbot, but to the many gentlemen who have assisted me with the loan of valuable apparatus, with counsel, with voluntary service and with painstaking observation, to one and all of whom I desire to express my obligations.
E Abstract of a lecture delivered at the Medical Graduates’ College and Polyclinic, and printed in the Lancet of May 19.
This lecture is devoted to a description of the parasite and of its life cycles. The existence of a parasite in malarial disease has been suspected for a long time, but only very recently have we had absolute assurance that such a parasite exists. Some time in the thirties Meckel described in the human blood certain black particles which he found in leucocytes and in certain pale, leucocyte-like bodies the nature of which he did not know. When he saw these bodies he certainly saw the malarial parasite. His observations were repeated and extended in the forties and the fifties by Frerichs and Virchow, and they, too, undoubtedly saw the malarial parasite. But it is one thing to see and quite another to recognize; discovery is recognition.
The discoveries of Laveran, Golgi, Marchiafava, Bignami and others resulted in considerable knowledge of the life history of the malarial parasite and of the correspondence between its life cycle and the clinical cycle of the disease. Laveran discovered the parasite; Golgi described the cycle of the tertian and quartan forms; the others added new data, especially concerning the more malignant parasites. The malarial parasite in its mature form has the appearance—I shall take the tertian parasite as a type—of a mass of pale protoplasm occupying practically the whole of the red blood corpuscles. Scattered through this mass of protoplasm are a number of black specks or little rods of intensely black pigment. Later in the life of the parasite a peculiar thing happens: all these little specks of black pigment concentrate usually towards the center of the organism whilst the pale protoplasm arranges itself into little spherules, the whole constituting what is known as the ‘rosette body.’ Later in the life of the parasite the surrounding blood corpuscle breaks away and this rosette body floats free in the liquor sanguinis and then breaks up into its constituent spores, setting free at the same time the black pigment clump. Phagocytes attack many of these free spores and probably absorb most of them, as well as the little pieces of pigment. The result is the pigmented leucocyte, so characteristic of malarial blood. A few of the spores escape and in virtue of some peculiar faculty, which is not at present311 understood, enter fresh blood corpuscles and appear there as pale specks in the hæmoglobin. These pale specks, if watched in perfectly fresh blood, are seen to be possessed of very active amœboid movement. They throw out pseudopodia in various directions and wander about through the hæmoglobin of the corpuscle. After a time they increase in size by assimilating the hæmoglobin. By and by there appear somewhere in the parasite those specks of black pigment which we saw in the mature animal. Later they increase still further in size until they come to occupy half, and finally nearly the whole, of the blood corpuscle. Again there is concentration of pigment and the formation of little sporules. This is the cycle, as described by Golgi, of the tertian and quartan parasite. The cycle of the tropical or æstivo-autumnal parasite corresponds in plan almost exactly with that of the quartan and ordinary tertian parasite.
It was found that the life cycles of these parasites ran parallel with the clinical cycle of malarial disease. It was found that when the parasite had arrived at maturity the apyretic interval in an ague was about to conclude, and that when the parasite had arrived at the sporulating stage the patient had entered on the shivering stage of his fever. During that and the succeeding hot and sweating stage the spores had entered the red blood corpuscles, and when the parasite had ensconced itself in the red blood corpuscle and begun to grow, the fever had come to an end. It was found in tertian fever that the cycle of the parasite took forty-eight hours to complete, exactly the length of the cycle of the clinical phenomena. In quartan fever the cycle took seventy-two hours, exactly the length of the clinical cycle of that form of malarial disease. In the malignant or tropical fevers there was found to be a similar correspondence between the cycle of the parasite and the cycle of the disease. It was found that with each recurring paroxysm of fever there was a renewal of the life of the parasite, and that in this way the life of the parasite was continued from period to period and from cycle to cycle for weeks or even, especially in the case of quartan malaria, for months. Now this explains very well the way in which the malaria parasite contrives to maintain its existence in the human body, but it does not explain how it passes from host to host, neither does it explain certain appearances that Laveran and everybody else who has studied the subject have witnessed. In malarial blood you sometimes see that peculiar body, the flagellated body, which I have already alluded to as consisting of a sphere surrounded by from one to six or seven long tentacles or arms in a state of continual agitation. Neither does it explain the peculiar crescent-shaped body which also so pointedly arrested Laveran’s attention.... Golgi’s scheme leaves the passage of the parasite from host to host and also the nature of these two bodies unexplained. What relation have312 these two bodies to the life of the parasite? Their nature and purpose do not receive any illumination from Golgi’s theory. You will find in all forms of malarial infection, if you look enough, the flagellated body; but, strange to say, you will not find it in malarial blood immediately after it is withdrawn from the body. It is only after an interval of minutes, perhaps a quarter of an hour, after the blood is withdrawn that these flagellated bodies appear. Whence do they come? If you make a preparation of malarial blood from a patient by pricking the finger and spreading a little of the blood on a slide, fixing it immediately with heat or alcohol and staining it, you will never see any of these flagellated organisms. But if the slip be kept moist and in a warm temperature for half an hour and then stained, the flagellated bodies will be seen, proving that they develop only after the escape of the parasite from the human body. Such a fact is very interesting and obviously has some significance in connection with the life of the parasite. Whence, I ask, come these flagellated bodies? If one of the crescent-shaped bodies is observed continuously, the following changes of shape may often be observed: It becomes shorter, loses its crescent shape and gives off flagella, which may break off and swim about by themselves. When they come in contact with a blood corpuscle they straighten themselves out and indulge in a peculiar vibratory movement, as if endeavoring to penetrate the corpuscle.
Many years ago King, in America, and others too numerous to mention suspected that the mosquito had something to do with malaria, but in what way they could not say. Not only civilized observers had this suspicion, but the savage natives of certain tropical countries had the same idea. Koch tells us that certain natives of German East Africa who lived in a mountainous, and therefore non-malarial, part noticed that when they descended to the malarial regions on the coast they acquired a fever which they called ‘mbu.’ They said that they were bitten there by certain insects which they also called ‘mbu’—mosquito or gnat. They give the same name to the mosquito and to the fever, therefore obviously these savages associate the insect and the fever as cause and effect. Peasants in certain parts of Italy have the same idea, believing that the bite of the mosquito may be followed by the development of malarial fever.
Laveran, some years ago, in one of his numerous works on malarial fever, said that possibly the malarial parasite was cared for by the mosquito in the same way that the latter cares for the filaria of the blood. He did not, however, formulate a definite theory on the subject.
In 1894 I was engaged in working at malaria, following out Golgi’s work and that of other Italians. I was particularly struck by the phenomena of exflagellation and more particularly by the fact that it313 occurred only when the blood had been removed from and was outside the human body. I reasoned that if this exflagellation occurs only outside the body, the purpose of the flagellated body must lie outside the human body, and that therefore the flagellated body must be the first phase of the malarial parasite outside the body, must be the first step that the malarial parasite takes in passing from one human host to another. There seemed to me to be a sort of logic in this. But how was the malarial parasite to pass from one human being to another? It was not provided while inside the human body with any organs of locomotion or penetration; as far as we know the parasite is never extruded in the excreta, neither does it habitually escape in hæmorrhages. Therefore, the idea of a spontaneous escape of the parasite from the human body had to be dismissed. I therefore concluded that some extraneous agency must remove the parasite from the human body, so as to afford the opportunity for this flagellation which I had concluded must constitute the first step in its extra-corporeal life. In casting about for an organism which could effect this removal I, for many reasons similar in some respects to those that influenced the savage African, the Italian peasant, King, Laveran and others, came to the conclusion that the medium of removal and transit must be the mosquito. I was so impressed with the probabilities of this double hypothesis and with its extreme practical value, should it prove to be correct, that I endeavored to leave England for a time and to visit British Guiana or some such suitable malarial country where I might work out the idea. Unfortunately, that could not be accomplished, so I published my theory in the hope that it would appeal to someone who might enjoy the opportunities denied to me. At that time Surgeon-Major Ross was at home from India and we had many conversations on the subject. I described to him my hypothesis, the probabilities of which and the possibilities of which powerfully appealed to his highly logical and practical mind. He undertook, when he returned to India, to do his best either to establish or confute it. Accordingly he set to work in India experimenting with mosquitoes and malaria.
Ross was stationed in Secunderabad, in the south of India, where there was abundant opportunity for experimental work—plenty of patients and plenty of mosquitoes. He got patients with crescent parasites in their blood and he got mosquitoes to bite them. He found that in the course of a few minutes after the blood had entered the insects’ stomachs the crescent parasites proceeded to the formation of sphere and flagellated body. But he got no further. This experiment was repeated hundreds of times. Many of his preparations were sent to me, and I could confirm from them the accuracy of his statements on the subject. Ross was encouraged, for obviously we were on the314 right track. One day Ross, whose station had in the meantime been changed, caught some mosquitoes which had been feeding on a patient the subject of tertian malaria. He kept the mosquitoes and after a few days dissected them. He took the stomach out and placed it on a slip with a little salt solution, covered it with a cover-glass and examined it with a microscope. He was gratified to find lying amongst the transverse and longitudinal muscular fibres a number of spherical bodies, very sharply defined, and including a great many grains of intensely black pigment exactly like those of the malaria parasite. Ross was at once struck with the similarity. After years of labor he believed he had at last seen the malaria parasite in the tissues of the mosquito, where we reasoned it ought to be; and he was right. At a subsequent experiment on the malarial patient he found exactly the same bodies, and on dissecting several mosquitoes at different intervals of time he found that the parasite, which originally was six micro-millimetres in diameter only, grew to sixty or eighty micro-millimetres, each parasite, notwithstanding its growth and the lapse of time, still containing the peculiar and most characteristic black pigment. Ross was now quite sure that he had found the extra-corporeal phase of the malarial parasite. Some of these preparations he sent home. I examined them and showed them to a number of friends in London familiar with the malarial parasite; they agreed with me, as Laveran also did, in believing that probably this indeed was the long-sought-for extra-corporeal phase of the malarial parasite. Ross at that time had great difficulty in getting opportunities for experiment on the human subject and in procuring proper mosquitoes. He found that the mosquitoes in which he had discovered these pigmented bodies were of a different species to those on which he had formerly experimented, and that in this circumstance lay the explanation of his lack of success earlier as well as the secret of his ultimate success. Failing to get sufficient opportunity for experimenting on human malaria he turned to bird malaria. He found that the sparrow of Calcutta, in a large proportion of instances, contained in its blood a malaria-like parasite. Ross procured a number of infected sparrows and let loose upon them a number of mosquitoes of a species belonging to the genus culex. These mosquitoes, after from one to ten days, he dissected and examined their stomachs. He found in the stomach-wall pigmented bodies exactly similar to those which he found in the stomach-walls of mosquitoes fed on human malarial blood. He found that they increased in size and in a week or ten days grew from six to eighty micro-millimetres in diameter. When they became of considerable size they protruded like warts from the surface of the insect’s stomach and were included in a very definite capsule. At this stage the capsule was filled with a vast number of very minute rod-like bodies. These capsules, which now projected into the315 body cavity of the insect, being over-distended, ruptured and discharged the rod-like bodies into the body cavity of the mosquito. For a time Ross could get no further than this. He could not find what became of the rod-like bodies. One day, in dissecting the head of a mosquito, he encountered two small trilobed glands the ducts from which united to form a main duct. The glands lay on either side of the head and the common duct he traced to the base of the proboscis of the mosquito. This was the salivary gland of the mosquito. He found that the cells of the gland contained rod-like bodies exactly like those which he had found inside the parasitic capsules in the stomach-wall. He concluded that somehow these ‘germinal rods’ (for so he called them) had managed to find their way into the salivary gland of the mosquito. It immediately occurred to him that this might be the route by which the parasite escaped from the mosquito into its vertebrate host. No sooner had the idea occurred to Ross than he put it to the test of experiment. He selected a number of sparrows in whose blood he satisfied himself that there were no parasites and let loose upon them a number of mosquitoes which he had already infected with malarial parasites. He found after a week or ten days that the sparrows which were experimented upon sickened and many of them died; and in their blood he found the malarial parasite.
We now understand why the flagellated body is developed outside the human host: because its function lies outside the human host. We now understand why the flagella break away and enter the granular sphere: they impregnate it and start it on the road of development. We now understand why MacCallum’s vermicule is beaked and endowed with powers of locomotion and penetration: that it may approach and penetrate the stomach of the mosquito. And we now know why the sporozooites, the ‘germinal rods,’ enter the mosquito’s salivary gland: that they may be injected into vertebrate issue and so pass the parasite from vertebrate to vertebrate.
This is one of those fairy tales of science which people are inclined to doubt, but any one who has worked at the subject and taken the trouble to go through the long series of preparations which have been sent home from India can not for a moment have the slightest doubt that what Ross stated was absolutely true, and that not only for bird but for human malaria. So soon as the idea got abroad that the key to the way in which the malarial parasite is propagated had been found the Italians immediately set to work with renewed vigor and with the utmost skill. Almost at once they demonstrated that what happened in the case of Ross’s sparrows happened also with the human subject: that the appropriate species of mosquito fed upon the human malarial subject and subsequently allowed to feed upon a non-malarial subject conveyed the malarial parasite and malarial disease, and that the appropriate316 species of mosquito belonged to the genus anopheles. There can not be the slightest doubt that the mosquito acts the part of transmitting agent as well as definitive host of the malarial parasite.
This is a piece of knowledge of the utmost importance to mankind, for we know that malarial disease in tropical countries—which, after all, in the future will be the most important parts of the world, seeing that they can produce more food than temperate countries and can therefore support a larger population—causes more deaths and more disposition to death by inducing cachectic states predisposing to other affections than all the other parasites affecting mankind put together. We know now in what way this parasite is acquired. Depend upon it, in time, in virtue of this knowledge, we will get enormous power over the disease and sooner or later we will be able to prevent the infection of man by the parasite. It is only a question of study and the application of the knowledge already acquired, only a question of money and perseverance and a little ingenuity, and these results will come. It may not be in ten years or twenty years, but sooner or later the energies of a considerable portion of scientific mankind now being expended in endeavoring to devise means for preventing the infection of men with the malarial germ by the mosquito will bear valuable fruit.
You can readily understand that it is of great importance to be able to recognize the special species of mosquito which convey malaria. The effective species as regards human malaria belong to the genus anopheles; species of the genus culex are effective in the case of sparrow malaria. Fortunately, these two genera are easily recognized even by the amateur zoölogist. If you find a mosquito clinging to the wall or other surface you can tell which genus it belongs to by its posture. If the body is stuck out nearly at right angles to the surface on which the insect is resting, it is an anopheles. If the body is almost parallel to the surface, it is a culex. There is another test which is easily applied if you have a pocket lens; in culex the two organs known as palpi are rudimentary and very short; whereas in anopheles those organs are almost as long as the proboscis. It should be remembered that the male mosquito is not a blood-sucker and therefore is not dangerous. It is the female anopheles which transmits the disease. The mosquito larvæ inhabit stagnant or slow-running water. If a mosquito larva be found with its head downwards, the body hanging at right angles to the surface of the water, it is a culex; if the body lies parallel to the surface of the water, it is an anopheles. There are other points of difference with which I need not now trouble you; those referred to suffice for diagnosis between the innocuous and the dangerous mosquitoes.
The facts regarding the malaria parasite which I have described are of great importance for many reasons. First, because they help317 us to understand the pathology and etiology of malaria. Secondly, they help us in diagnosis. Thirdly, our knowledge of the parasite is invaluable in directing treatment. Lastly, a knowledge of the life-history of the malarial parasite is of extreme value for the prevention of malarial disease, for could we by mechanical or other arrangements prevent the mosquito attacking the human body, we could prevent the malarial parasites from entering the human body; or if we could abolish the mosquito by drainage or other means from a country, then we might be sure that we would abolish the malaria of that country also.
Attempts are being made to solve these practical problems. At the present moment such attempts are being actively made in Rome by Professor Celli and elsewhere by others. I have no doubt that in the course of a few years we shall get some very valuable results in this direction and that, thanks to this new-born knowledge about the malarial parasites, better times are rapidly approaching for malarial countries.
Among the general laws of physical science, none seems more firmly established than that of the conservation and correlation of energy; according to this the various forms of energy that constitute the domain of experimental physics, heat, light, electricity, magnetism and chemical action, have reciprocal dependence and “can not originate otherwise than by devolution from some preëxisting force,” or rather energy. That motion is convertible into heat, heat into light and both the former into electricity are phenomena familiar to every one who uses incandescent bulbs or rides in a trolley, and we do not usually recognize any production of light unaccompanied by heat. True, the little fire-fly is possessed of a mysterious power that enables it to emit light without enough heat to affect Langley’s most sensitive bolometer, but the eminent Secretary of the Smithsonian has to admit that the “cheapest form of light” is produced by “processes of nature of which we know nothing.” This little understood property called phosphorescence is shared by many living organisms, both animal and vegetable, as well as by substances of the mineral kingdom; to the former belong coelenterates, mollusks, crustacea, fishes and insects, and decaying wood, certain mushrooms, etc.; to the latter the Bologna stone, so-called, and the commercial article called ‘Balmain’s paint.’
In the case of the mineral substances, barium or calcium sulfids and the like, the light-giving power is not an innate property, but is set in operation by exposure to the energy of sunlight, the light of burning magnesium or to some other source of actinism; moreover, the power thus acquired by insolation is a fugitive one, the substances exercising it after three or four hours become ‘dead’ and lose their activity. Excepting then these living beings and these phosphorescent bodies, light as commonly known to us is always correlated with heat; within the last four years, however, discoveries have been made in France that seem to modify the position taken by philosophers and to necessitate new views concerning the manifestations of that energy with which the universe is endowed. A group of French savants have found mineral substances that apparently give out light perpetually without any exciting cause, realizing the dream of the alchemists—a perpetual lamp consuming no oil. These substances also emit rays having the penetrating properties of X-rays, other rays affecting a photographic319 plate, and fourthly, rays causing air to become a conductor of electricity. The history of these discoveries can be briefly given.
Röntgen’s discovery of the rays that pass through metals and solids opaque to light was made in 1895, and in the following year, Becquerel, a distinguished French academician, discovered that salts of the metal uranium (substances that had long been used in coloring china and glass) emit invisible radiations capable of discharging electrified bodies and of producing skiagraphic images on sensitive plates; he found that potassio-uranic sulfate emits rays that pass through black paper and give photographic impressions in the same way as Röntgen rays. This property is not limited to the brilliantly fluorescent uranic salts, but is shared by the non-fluorescent uranous salts, and is exhibited by compounds whether phosphorescent or not, whether crystalline, melted or in solution, as well as by the metal itself. The permanence of this activity is amazing, substances kept in a double leaden box more than three years continuing to exert the power.
Shortly after the announcement by Becquerel, experimenters found that other substances have the power of emitting ‘Becquerel Rays,’ such as calcium and zinc sulfids and compounds of thorium. In 1898 Mme. Sklodowska Curie, working in the laboratory of the Municipal School of Industrial Physics and Chemistry in Paris, devised a special apparatus for measuring the electrical conductivity of the air when under the influence of ‘radio-active bodies,’ and by its means studied the behavior of pitchblende (uraninite), and of other uranium minerals; finding that some specimens of pitchblende had three times as much energy as uranium itself, she came to the conclusion that the peculiar property is due to some unknown body contained in the minerals and not to uranium. Examining the mineral with the aid of her husband, the two found a substance analogous to bismuth, four thousand times stronger than uranium, which was named ‘Polonium,’ in honor of the native land of Mme. Curie. In December of the same year, the lady received the Gegner prize of 4,000 francs awarded by the Academy of Sciences, as a substantial appreciation of her discovery, and later in the same month Mme. and M. Curie announced that they had found a second body in pitchblende, which they named ‘Radium.’ More recently, M. Debierne, working under the auspices of Mme. Curie, has discovered a third body, which he calls ‘Actinium,’ an unfortunate appellation because ‘actinium’ has already been used for an element announced by Dr. Phipson and since discarded.
These three ‘radio-active’ substances do not possess identical properties; their rays are unequally absorbed and are differently affected in a magnetic field; moreover radium emits visible rays, while polonium does not. Nor have they the same chemical affinities; polonium belongs to the bismuth group, radium to the barium and actinium to the320 titanium series. They have not been separated perfectly from their analogues, and consequently their chemical properties and the actual intensity of their physical activities is very imperfectly known. The difficulties of securing even small quantities of crude materials are enormous; Fritz Giesel obtained from one thousand kilograms of raw material only fifteen grams of active compounds, and Mme. Curie, operating on half a ton of the residues of uranium from a chemical manufactory, got about two kilograms of barium chloride rich in radium, but the percentage of active substances in these mixtures is unknown.
Radium is spontaneously luminous, and all the bodies emit rays that excite phosphorescence in gems, fluorite and other minerals; they communicate radiant energy to inactive substances, and they exert chemical action, transforming oxygen into ozone and producing changes in the color of glass and of barium platino-cyanid.
Through the enterprise and liberality of the Smithsonian Institution, and by the courtesy of Professor Langley, I have enjoyed the opportunity of studying small specimens of these rare and costly substances; they comprised ten grams of ‘radio-active substance’ prepared by a manufacturing chemist of Germany and smaller quantities of ‘radium’ and of ‘polonium’ from Paris. On removing the wrappings of the German specimens in a dark room, they were seen to emit greenish-white light that gave to the enveloping papers a peculiar glow, similar to the fluorescence produced by Röntgen rays. Simple tests of the radium showed that it gave the usual reactions of barium; on boiling it with water it lost its luminosity, but on heating to dull redness this property returned in the dark. It also caused a barium platino-cyanid screen to fluoresce.
Experiments to test the actinic power of these bodies gave interesting results; on exposing sections of photographic plates, at distances of five inches, from two to twelve minutes, bands were obtained varying in intensity with the duration of action. By exposing sensitive plates behind negatives to the radiant materials from two to three hours, excellent transparencies were secured; on substituting Eastman’s bromide paper good prints were obtained.
The penetrating power of the rays emitted permits the production of skiagraphs; the plates were enveloped in Carbutt’s black paper (impermeable to light), and on them were laid pieces of tinfoil cut in openwork pattern; after one hour’s exposure negatives were secured plainly showing the pattern. Analogous experiments were carried on with the specimens from Paris, but they were only one fifth as strong in effects; that labelled ‘polonium sub-nitrate’ had positively no action on the plates used.
The primary source of the energy manifested by these extraordinary substances has greatly puzzled physicists, and as yet remains a mystery.321 Mme. Curie, speculating on the matter, conjectured that all space is continually traversed by rays analogous to Röntgen rays, but far more penetrating, and not capable of being absorbed by certain elements of high atomic weight, such as uranium and thorium. Becquerel, reflecting on the marvellous spontaneous emission of light, said: “If it can be proved that the luminosity causes no loss of energy, the state of the uranium is like that of a magnet which has been produced by an expenditure of energy and retains it indefinitely, maintaining around it a field in which transformation of energy can be effected; but the photographic reductions and the excitation of phosphorescence require an expenditure of energy, of which the source can only be in the radio-active substances.” Somewhat later, Becquerel hazarded the opinion that the radiation is composed at least in part of cathodic rays; but these have been proved to be material, hence the induced activity must be caused by material particles impinging upon the substances excited. This materialistic theory seems to be confirmed by the results of ingenious experiments made by Mme. and M. Curie; they placed a sensitive plate beneath a salt of radium supported on a slab of lead, in the vicinity of an electro-magnet. Under these conditions, when the current was passing, the rays emitted were bent in curved lines upon the sensitive plate, making impressions.
It may be objected, says a French writer, that the materialistic theory requires us to admit actual loss of particles of matter, nevertheless the charges are so feeble that the most intense radiation yet observed would require millions of years for the removal of one milligram of substance.
While writing these lines, we have news of experiments that seem to throw doubt on the elementary character of these radio-active bodies; Bela von Lengyel, of Budapest, claims to have prepared the so-called ‘radium’ synthetically. By fusing with the heat of electricity uranium nitrate mixed with a small percentage of barium nitrate, and treating the mass with acids, he obtained a substance that gives out actinic rays, Röntgen rays, excites platino-cyanid screens and causes air to conduct electricity; in short, the Hungarian chemist gets material possessing all the properties characteristic of the ‘element’ announced by Mme. Curie.
Admitting that radio-active bodies can be manufactured to order, are we any nearer explaining their mysterious powers?
Speculations as to the future history and applications of these wonder-working bodies press upon even the dullest imagination; if a few grams of earth-born material, containing only a small percentage of the active body, emit light enough to affect the human eye and a photographic plate, as well as rays that penetrate with X-ray power, what degree of luminosity, of actinism and of Röntgenism (if the term322 may be allowed), is to be expected from an hundred weight of the quintessence of energy purified from interfering matter? And to what uses is this light-generating material to be applied? Are our bicycles to be lighted with discs of radium in tiny lanterns? Are these substances to give us the ‘cheapest form of light?’ Are we about to realize the chimerical dream of the alchemists?
Seriously, in what direction is profound study of these substances going to lead us? Will it not greatly extend our knowledge of physical manifestations of energy and their correlation? In what corner of the globe will be found the cheap and convenient supply of the raw material yielding the radio-active bodies? Will not chemists be obliged to re-examine much known material by laboratory methods conducted in the dark? Many of us have worked up pounds of pitchblende to extract the uranium oxids, and in so doing have poured down the waste-pipe or thrown into the dust-bin the more interesting and precious bodies.
Whatever the future may bring, scientists are deeply indebted to Becquerel and to Mme. and M. Curie for placing in their hands new methods of research and for furnishing a novel basis for speculation destined to yield abundant fruits.
Washington was a surveyor and explorer before he entered upon the fields of war and statecraft, and his honesty of purpose, sincerity of action and accuracy of statement and method, so manifest throughout his career as a soldier and statesman, are found also in the earlier record. At the age of sixteen he crossed the Blue Ridge on horseback and made a series of successful surveys in the Shenandoah valley, overcoming physical obstacles with the method and system of a modern scientist. At twenty-two he led a party into the wilderness of the valley of the Ohio to treat with the French and Indians. He then became acquainted with the great resources of the interior, and saw that the valleys of the James and Potomac afforded unusual facilities for lines of transportation for the trade ‘of a rising empire.’ In 1754 he reported in favor of a scheme of communication between the Atlantic states and the great west. Sixteen years later he suggested that the project of opening up the Potomac be ‘recommended to public notice.’ The idea contained in the Potomac scheme was of far-reaching import, and only the present generation can fully realize its significance.
Washington was not only the first to map and recommend the general route of the great highways called the National Pike and the Chesapeake and Ohio Canal, which are now in truth ‘becoming the channels of conveyance of the extensive and valuable trade of a rising empire,’ but he was also the first to predict the commercial success of that route through the Mohawk valley which was afterwards taken by the Erie Canal and the New York Central Railroad.
One hundred and fifteen years ago he asked: “Would it not be worthy of the wisdom and attention of Congress to have the western waters well explored, the navigation of them fully ascertained and accurately laid down, and a complete and perfect map made of the country.... The advantages would be unbounded, for sure I am that nature has made such a display of her bounties in those regions that the more the country is explored the more it will rise in estimation, consequently greater will the revenue be to the Union.” Again he declared, “I shall not rest contented until I have explored the western country and have traversed those lines which have given bounds to a new empire.”
Washington did not do this as fully as he wished, but his ambition has been and is being realized through the medium of hundreds of enterprises under both national and private encouragement. The result of a trip made in the fall of 1784 was the real historic beginning of the Potomac enterprise. On his return he wrote to Benjamin Harrison, Governor of Virginia, “I shall take the liberty now, my dear sir, to suggest a matter which would mark your administration as an important era in the annals of this country if it should be recommended by you and adopted by the Assembly.” He reached far out for those days, assuming Detroit as a point of departure for the trade of the northwest territory. His confidence in the practical abilities of the American people is shown by the remark, “A people who are possessed with the spirit of commerce, who see and will pursue their destinies, may achieve almost anything. No person who knows the temper, genius and policy of this people as well as I do can harbor the smallest doubt.”
In urging the Potomac scheme, he324 later asked that commissioners be appointed to make a careful survey of the Potomac and James rivers to their respective sources, and that a complete map of the country intervening between the seaboard, the Ohio waters and the Great Lakes be presented to the people. “These things being done,” he says, “I shall be mistaken if prejudice does not yield to facts, jealousy to candor and finally, if reason and nature, thus aided, do not dictate what is right and proper to be done.”
He introduced his plan to the notice of Congress, thus making the first suggestion to that body of the policy of national improvements which the present generation is carrying on, as well as of the policy of exploration and national surveys to which our Government so firmly adheres. To-day the Government is carrying forward surveying work by means of the largest and most thoroughly equipped organizations in existence, and thus is Washington honored.
The scientific men of to-day owe to Washington profound respect and gratitude for the scientific spirit he cultivated in his work. The Government once established on so high a plane, it necessarily followed that all true science should be encouraged and be enlisted in the development of the citizen and of the material resources of the nation.
Charles D. Walcott,
U. S. Geological Survey,
Washington, D. C.
The leading article of the June number of the Century Magazine is entitled “The Problem of increasing Human Energy,” and is written by Nikola Tesla. Mr. Tesla offers the reader some naive verbal analogies between the causes of human progress and the ‘energy’ of theoretical physics, and a eulogy of a number of inventions which he expects to make. He intersperses these with sundry remarkable statements such as, “our own earth will be a lump of ice;” “Though this movement is not of a translatory character, yet the general laws of mechanical movement are applicable to it;” “That we can send a message to a planet is certain, that we can get an answer is probable;” “It is highly probable that if there are intelligent beings on Mars they have long ago realized this very idea [the transmission of electrical energy for industrial purposes without wires], which would explain the changes on its surface noted by astronomers.” (The italics are our own.)
Mr. Tesla’s doctrine of human energy is in some ways as original as the inventions and discoveries which he expects to make. Each of us is, he says, a part of a unitary whole, ‘man.’ “This one human being lives on and on.... Therein ... is to be found the partial explanation of many of those marvelous phenomena of heredity which are the result of countless centuries of feeble but persistent influence.” Now we may “assume that human energy is measured by half the product of man’s mass with the square of a certain hypothetical velocity ... the great problem of science is, and always will be, to increase the energy thus defined.... This mass is impelled in one direction by a force F, which is resisted by another partly frictional and partly negative force R, acting in a direction exactly opposite, and retarding the movement of the mass.”
Unhappily Mr. Tesla in his enthusiasm to progress to recommendations of religion, vegetarianism, the old régime for women and the artificial preparation of nitrogen compounds, neglects to state which direction is the proper one for the human mass to follow, north, south, east, west, toward the moon or Sirius or to Dante’s Satan in the centre of the earth. Nor does he explain how ‘enlightenment’ makes the mass of human bodies go in an exactly opposite direction to that toward which ‘visionariness’ impels them, nor reveal why, if his account be true, he and a ‘visionary’ can walk in the same direction.325 Of course the whole notion that the ‘velocity’ of the human ‘mass,’ i.e. the space it traverses in a given time, has any connection with human progress or is of any value to anybody or anything, is absurd.
Mr. Tesla has enjoyed considerable, excellent repute as a gifted student of certain electrical phenomena and one expects a good deal from his “electrical experiments, now first published.” Mr. Tesla, too, expects a good deal from them. It would take too long to even note here all the important scientific discoveries which Mr. Tesla expects to make or all the benefits which he expects to thereby confer upon mankind in general and in particular upon those who exploit his inventions. Some samples may be given. War will be rendered harmless by being reduced to a sort of game between ‘telautaumata,’ machines which behave “just like a blind-folded person obeying instructions received through the ear,” any one of which is “enabled to move and to perform all its operations with reason and intelligence.”
Says Mr. Tesla: “I purpose to show that, however impossible it may now seem, an automaton may be contrived which will have its ‘own mind,’ and by this I mean that it will be able, independent of any operator, left entirely to itself, to perform, in response to external influences affecting its sensitive organs, a great variety of acts and operations as if it had intelligence. It will be able to follow a course laid out or to obey orders given far in advance; it will be capable of distinguishing between what it ought and what it ought not to do, and of making experiences or, otherwise stated, of recording impressions which will definitely affect its subsequent actions. In fact, I have already conceived such a plan.”
Inasmuch as the interest in this telautomatic warfare is to be purely æsthetic, it would seem as if international bull-fights or kite-flying or spelling matches or potato-races might do as well, and have the added advantage of leaving Mr. Tesla’s expectations free to wander among the following prospective discoveries.
New sources of energy, Mr. Tesla thinks, may be opened up, such as a wheel which shall perform work without any further effort on our part than that of constructing it. “Imagine a disc of some homogeneous material turned perfectly true and arranged to turn in frictionless bearings on a horizontal shaft above the ground. This disk, being under the above conditions perfectly balanced, would rest in any position. Now, it is possible that we may learn how to make such a disk rotate continuously and perform work by the force of gravity without any further effort on our part.... To make the disk rotate by the force of gravity we have only to invent a screen against this force. By such a screen we could prevent this force from acting on one half of the disk, and the rotation of the latter would follow.”
Into further particulars concerning the nature of such a screen Mr. Tesla does not enter, though it would seem a matter well fitted to engage his peculiar gifts. The ‘screen against gravity’ idea has already entered into a popular story, but scientific men have probably not given it much consideration.
By producing a ‘sink’ or reservoir of a low temperature, thereby inducing the heat of the ambient medium to transform itself in part into other forms of energy (e.g. electrical), Mr. Tesla hopes to “get any amount of energy without further effort” beyond the amount needed to create the ‘sink.’ We should thus employ “an ideal way of obtaining motor power,” and incidentally rebuke the narrow-minded physics of Carnot and Lord Kelvin.
By means of his electrical oscillator Mr. Tesla has satisfied himself that he can transmit electrical energy in large quantities without wires. He expects that this can be done to great economic advantage. Then would come the golden age. “Men could settle down everywhere, fertilize and irrigate the soil326 with little effort, and convert barren deserts into gardens, and thus the entire globe could be transformed and made a fitter abode for mankind.”
The golden age figures largely in Mr. Tesla’s article; he offers us all that is entrancing and wonderful. He is generous. We ask for the bread of definite facts of science and intelligible evidence, but he gives us the amethyst and topaz and diamonds of an ambient medium doing all our work and the atmosphere transporting all our motive power and the tyrant gravity held powerless by a screen, and Mr. Tesla correcting Lord Kelvin’s errors. Still amethyst and topaz and diamonds are only stones. They may dazzle the magazine reader, but they do not nourish the student of science.
The editorial department of the Century Magazine perhaps felt that these jewels were a bit too bright. We read there that “much that must seem speculative to the layman can take its proper place only in the purview of the scientist.” Some conservative scientists will feel like growling, “And much that must seem bosh to the man of science can take its proper place only in the purview of the editorial departments of popular magazines.” Leaving aside the present case, it is a fact that the same care which is exercised by editors to secure in their contributions excellence of style and syntax, a proper moral tone and freedom from advertisement of business ventures, is not exercised to secure accuracy in statements of fact or decent credibility in matters of theory. The editors apparently impute to their readers a desire to be entertained at all costs. They descend to a footing with the Sunday newspaper instead of trying to rise to the level of such scientific literature as Huxley or Tyndall gave us. They evidently often do not know science from rubbish and apparently seldom make any effort to find out the difference. They should at least submit their scientific literature to competent men for criticism and revision.
The general public is helpless before any supposedly scientific statement. It may judge vaguely by the standing of the paper or magazine or book containing it, by the name of the writer or by the general tone in which the article is written. But it cannot judge definitely by comparison with relevant facts or by critically examining the logic of the deductions, for the general public lacks both knowledge of the relevant facts and training in logical criticism. That a man should invent a microscope which will enable one to see objects a million times as small as can be seen with the naked eye seems no more questionable to the general public than that a man should cause unfertilized eggs to develop. Yet the first would be impossible while the second has been possible, probable, and still more lately proved. Guidance in scientific matters should be welcome if only for the protection thus given against fraudulent medicines, bogus inventions and nonsensical enterprises.
Physicist.
The memoirs presented to the Cambridge Philosophical Society on the occasion of the jubilee of Sir George Stokes, have been published in a stately volume by the Cambridge University Press. A year ago some four hundred men of science met at Cambridge to celebrate the fiftieth anniversary of the appointment of Sir George Stokes to the Lucasian professorship of mathematics, a chair held by Newton and a distinguished line of mathematicians. An official account of the proceedings, with a portrait of Professor Stokes, is given in the volume now issued. The seventy-two institutions sending delegates are arranged chronologically in the order of their foundation, and it is not unworthy of note that among the sixteen oldest institutions, the United States has five representatives, whereas Great Britain has thirteen universities and colleges younger than the Johns Hopkins University. The Rede lecture given by M. Alfred Cornu and entitled ‘La théorie des ondes lumineuses,’ is published in French, even the quotations from Newton’s ‘Opticks’ being translated into that language. M. Cornu states that by ‘une étude approfondie’ of the ‘Opticks,’ his lecture shows that Newton favored Descartes’s undulatory theory of light, rather than the emission theory usually attributed to him. The twenty-two memoirs that follow cover a wide range of subjects, nearly all of which have, however, a connection with the researches of Professor Stokes. They include three contributions from the United States, mathematical papers by Profs. E. W. Brown and E. O. Lovett, and a description by Professor Michelson of his echelon spectroscope.
In addition to this memorial volume, the Cambridge University Press, which is represented in America by The Macmillan Company, is at present publishing the collected papers of three eminent students of mathematical physics. The first volume of Lord Rayleigh’s ‘Scientific Papers’ contains seventy-eight contributions published from 1869 to 1881. The early papers show the influence of Maxwell, Lord Rayleigh’s predecessor in the chair of experimental physics at Cambridge, but it was apparently not until 1881 that he fully appreciated the importance of Maxwell’s electro-magnetic theory of light. The papers on acoustics were followed by the publication in 1877 of the classical work on the ‘Theory of Sound.’ Lord Rayleigh, at an early period, treated various optical subjects, including some of the phenomena of color vision. His explanation of the blue color of the sky and his treatment of the resolving power of telescopes are well known. The contributions on optics and acoustics have been continued to the present time, but they by no means limit his interests. There are important papers on hydrodynamics and mathematics, and longer and shorter contributions on a great range of subjects in mathematical physics, the science which at the present day is perhaps of supreme importance.
The second volume of Professor Tait’s ‘Scientific Papers’ contains those published since 1881. The first volume consisted of sixty papers, and this volume, which has followed with but little delay, adds seventy-three. As must be the case in collected papers, some are elaborate treatises while others fill only part of a single page; some are extremely technical while others were first published in the ‘Encyclopædia Britannica’ and the ‘Contemporary Review.’ Among the more elaborate papers are328 those on the physical properties of water contributed to ‘The Voyage of H. M. S. Challenger,’ on the kinetic theory of gases, on impact and on quaternions.
The third series just published by the Cambridge Press is the ‘Papers on Mechanical and Physical Subjects’, by Prof. Osborne Reynolds, of Owens College. The first volume contains forty papers from transactions and journals issued from 1869 to 1882. The most elaborate memoir is that on certain dimensional properties of matter in the gaseous state, which includes experiments on thermal transpiration of gases through porous plates and a theoretical extension of the dynamic theory of gas. Many of the papers, such as those on meteorological phenomena and the steering of vessels, are of popular interest. The Cambridge University Press is performing a work of the utmost value to science in undertaking the publication of these great volumes, and we can only regret that, in spite of the beginnings made at Johns Hopkins, Chicago, Pennsylvania and Columbia, American men of science have no such opportunities for the publication of their works as those afforded at Cambridge and Oxford.
That a large amount of popular interest centers in the study of tree life and all subjects incidental to forestry and horticulture is evidenced by the appearance of a second book on the subject under the title of ‘Our Native Trees and How to Identify Them’ (Scribners), by Harriet L. Keeler. The volume in question takes up the trees native of northern United States east of the Rocky Mountains, together with a few well-known foreign species which have become naturalized in this region.
The book opens with a key to the families of dicotyledonous species based upon leaf characters, and every species receives not only a full technical description, but also comes in for interesting comments upon habit and general ecological relations. Numerous drawings and half-tones add to the accuracy and clearness of the descriptions. It is not too much to say that the photographic reproductions surpass in beauty and presentation of detail any recent botanical publication, and the venation of leaves is shown in most instances by this method quite as well as it might be done by means of pen and ink sketches. The value of the descriptions is heightened by the inclusion of notes of economic interest. It is not unexpected that some errors should creep into the discussions on almost all phases of botany which are interspersed throughout the volume.
The appearance of a new botanical dictionary is most timely, and it is fortunate that the task of its preparation should be undertaken by such a skilful bibliographer as Mr. B. D. Jackson. His ‘Glossary of Botanical Terms’ (Lippincott) contains fifteen thousand words, or three times as many as have been included in any previous work of this character. This is indicative of a most energetic pursuit of investigations in all departments of the subject, and also of a lamentable tendency to the coinage by botanists of new and unnecessary terms upon the slightest pretext. A legitimate factor in the increase of the contents of such a work consists in the inclusion of words in common use which take on a technical meaning in botany; such, for instance, as altitude, abnormal, abrupt, absolute, accidental back, etc.
Derivations are given, but the history of the terms has not been attempted. According to the author, ‘anlage’ may be variously rendered as rudiment, inception or primordium. ‘Chlorophyll’ receives the double consonant at the end of the last syllable against the popular extra-botanical practice. Regarding ‘medullary’ the author says: “I have given the accent as it is always spoken (medul’-lary) though all of the dictionaries (botanical?) accent it as med’-ullary except Henslow’s.” In this the author had in mind the practice among his insular colleagues only, since the latter pronunciation329 is given in the Standard, Century and Webster’s Dictionaries and is followed by nine tenths of the American botanists. “Mycorhizome = mycorrhiza-like structures in Corallorhiza and Epipogum roots,” and “Mycorrhiza = symbiotic fungi on the roots of plants, prothallia, etc.,” are not only incongruous with orthography and botanical fact, but also with the usage of all recent writers on this subject.
While many other errors of this character could be adduced, the general value of the book is scarcely lessened, and it will be of the greatest service to the working botanist, not only in raising the general literary tone of his writings, but also in placing at his command a choice of all of the established terms dealing with any phase of the subject; an aid which will be greatly conducive to increased accuracy of statement.
A decade since, the majority of the botanists engaged in the study of the distribution of plants on this continent, as well as the strict systematists, were quite unanimously of the opinion that the territory within the boundaries of the United States had been quite thoroughly explored, and that the task of the collector are well-nigh done. Despite this discouraging conclusion a few enthusiastic workers have not intermitted their labors in a more critical consideration of the floras of the newer and less thickly settled regions, with the result that scores and hundreds of new species have been brought to light each year, and the awakening interest in the subject promises a re-exploration of the great West.
A striking example of the results awaiting the student in this line is afforded by Dr. Rydberg’s ‘Flora of Montana and the Yellowstone Park’ (New York Botanical Garden), which has recently appeared. Although the first collections of plants in this region were made by the Lewis and Clarke expedition nearly a century ago, Dr. Ryberg finds 163 new species and varieties in the 1,976 which he lists in this volume. Of this number 487 are found on both the eastern and western slopes of the continental divide, 268 on the eastern side only, 520 on the western side only, 42 of which are arctic and inhabit the high mountain summits, and 659 which have originated in the exact region under discussion. Seven hundred and seventy-six of the species listed were not included in Coulter’s ‘Rocky Mountain Botany,’ published a few years ago.
The symposium on the ‘Plant Geography of North American,’ to be given at the coming meeting of the American Association for the Advancement of Science, will do much to systematize investigations of this character and broaden the method of treatment accorded the subject in the future.
The ‘Biological Lectures from the Marine Laboratory of Woods Holl, 1899,’ make up a volume of about three hundred pages which represent fairly the present tendencies of biological investigation in this country. The most striking things about the lectures are the wide range of topics which they treat, and the first-hand quality of the subject matter in each case. This is most clearly seen by a careful reading of the text, but a mere enumeration of a few of the sixteen titles and lectures makes it fairly obvious. Thus, D. P. Penhallow writes on ‘The Nature of the Evidence Exhibited by Fossil Plants, and its bearing upon our Knowledge of the History of Plant Life;’ D. T. MacDougal writes on the ‘Significance of Mycorrhizas,’ Edward Thorndike on ‘Instinct,’ Herbert S. Jennings on ‘The Behavior of Unicellular Organisms,’ Alpheus Hyatt on ‘Some Governing Factors usually neglected in Biological Investigations,’ T. H. Morgan on ‘Regeneration,’ C. B. Davenport on ‘The Aims of the Quantitative Study of Variation,’ Jacques Loeb on ‘The Nature of the Process of Fertilization.’
To the professed scientist these lectures will furnish expert opinion on certain important topics; the general330 reader will find in them a presentation not too technical or detailed. Professor Loeb’s lecture, for example, is for such readers the best account yet given of his experiments in artificial fertilization.
The range and originality which characterize these lectures are really characteristic of the general work and spirit of the Woods Holl Laboratory. Few people realize the amount of research work which is done there from summer to summer. Yet last year there were seventy-one investigators there. Moreover, these represent a superior selection from among the instructors and students of the various colleges.
It is a symptom of a healthy, vigorous condition in biological science that the best workers of the country are glad to devote their vacation season to research, and it is highly creditable to the Woods Holl management that it offers them such attractive facilities. Similar summer laboratories are now being established in other parts of the country, and are to be reckoned with as very important factors in the progress of biology.
It is a somewhat surprising fact that among educated people of scientific training there prevails generally the greatest ignorance as to some of the most important problems of biology. We refer to those problems connected with the structure and functions of the animal and plant cell. Men who can understand and appreciate recent discoveries in astronomy, physics, chemistry and geology are usually wholly lost in cytology. In fact, in general writing or speech it is not safe to use this name without at once defining it, since it is commonly supposed to be a mispronunciation or a stupid misspelling of ‘psychology,’ while to most people nuclei, chromosomes, centrosomes and mitotic spindles are words without meaning, signifying nothing.
The reason for this is twofold: First, cytology is one of the newest of the biological sciences and it has but recently found its way into college curricula, and second, there have been few text-books or general works on this subject to which an intelligent layman could turn for information.
And yet, in spite of this fact, there are few fields of scientific work possessing more general interest than that of cytology. At the present day the greatest problems of biology are centered in the cell. Assimilation, growth, metabolism, reproduction, differentiation, inheritance and variation—these are at bottom cellular phenomena, the result of the structure and functions of cells. It is not surprising, therefore, that “all the searchlights of science have been turned upon the cell,” and that cell studies during the past ten years have received an amount of attention which is comparable only to that devoted to evolution under the stimulus of Darwin’s work.
Professor Wilson’s book on the cell,F the second edition of which has just appeared, is a work of more than ordinary interest, not only to the biologist, but to all persons who are interested in the general advance of science. Although there are several other good text-books of cytology which have appeared during the past five or six years, Professor Wilson’s book, in thoroughness of treatment, in philosophical insight, in clearness and forcefulness of style and in wealth and beauty of illustrations, easily surpasses them all.
F The Cell in Development and Inheritance. Edmund B. Wilson. Second Edition Revised and Enlarged. Columbia University Biological Series IV. New York and London, The Macmillan Co., 1900. Pp. xxi, 483 with 194 Figures in the Text. $3.50
It is impossible in this brief note to give any adequate summary of the volume or of the position of the author on questions of general interest; the subjects of the chapters, however, may serve to give some idea as to the scope of the work. After an introduction which gives a brief historical sketch of the cell theory and its relation to the331 evolution theory, there are taken up in successive chapters a general sketch of cell structure, cell division, the germ cells, fertilization of the ovum, the formation of the germ cells and the halving of their nuclei preparatory to fertilization, cell organs and their relations to each other and to the life of the cell, cell chemistry and cell physiology, cell division in its relation to the development of the egg, and finally, some theories of inheritance and development. In addition, there is appended an excellent glossary and a list of all the most important literature on the subject up to the current year.
While the work is undoubtedly intended as a reference book for investigators and advanced students in biology, being marked by the thoroughness of treatment of an original communication, it is yet so well written and so copiously illustrated as to make it not only intelligible but also intensely interesting to the general reader.
The most important recent book on education is undoubtedly ‘Education in the United States,’ a book prepared in connection with the educational exhibit of this country at the Paris Exposition. It consists of a series of monographs which cover all the important phases of educational endeavor in the United States. The two volumes include nearly a thousand pages, almost all of which present definite and reliable facts. Only rarely is there any indulgence in expressions of private opinion, and still more rarely is such opinion questionable. The editor is justified in his statement that the book is ‘a cross-section view of education in the United States in 1900.’ It will be of great value to the student of American institutions or of education in general, and should be of interest to any citizen who desires to be well informed about his country. The quality of the monographs will be evident from the list of the author’s names. For instance, those writing on higher education are Prof. A. F. West, of Princeton; Prof. E. D. Perry, of Columbia; President Thomas, of Bryn Mawr; Director Parsons, of the University of the State of New York; President Mendenhall, of the Worcester Polytechnic Institute, and Prof. H. B. Adams, of Johns Hopkins.
The conditions in the United States have been favorable to the development of geology. The varied forms of the land have offered abundant opportunities for research, whereas the practical value of the work has led to the establishment of surveys, the magnitude of whose contribution to geology is only known to special students. The Geological Society of America has about two hundred and fifty members, nearly all of whom are actively engaged in geological research, perhaps a larger number than in any other science. The U. S. Geological Survey is the center of this movement, and its great efficiency is in large measure due to Mr. G. K. Gilbert, now president of the American Association for the Advancement of Science. He was born in Rochester, N. Y., in 1843, and after graduating from the university in that city, acted for five years as assistant in the Ward Museum, where a number of eminent naturalists have been trained. He then became geologist in the Ohio Survey under Newberry, was engaged in the Wheeler and Powell Surveys, and has been geologist in the U. S. Geological Survey since its establishment in 1879. In the arid west, where the face of the earth is bare, Mr. Gilbert made the observations and discoveries in dynamical and physical geology which have done so much toward the making of the science of physiography. His monographs on the Henry Mountains and on Lake Bonneville, the name he gave to the ancient lake that once filled the Utah basin, are models, both in regard to their original discoveries and the methods of presentation. He has extended his studies to the basins of the Laurentian Lakes and to other regions, always with important results. Mr. Gilbert has been president of the American Society of Naturalists, the Geological Society of America and the Philosophical Society of Washington, and has received the Wollaston Medal of the Geological Society of London. His presidential address before the American Association will be given at the American Museum of Natural History, New York City, on the evening of June 26, his subject being ‘Geological Rhythm.’
The meeting of the American Association in New York City, opening as this issue of the Monthly is published, promises to be of more than usual importance. The preliminary programs of the different sections show long lists of valuable papers and promise the attendance of leading men of science from all parts of the country. A movement of interest is the increasing tendency of special scientific societies to meet in conjunction with the Association. No less than fifteen societies will this year hold their sessions at Columbia University, some of them joining with the sections of the Association, and others holding independent meetings. The members of these different societies have the advantage of the reduced railway rates and other arrangements which can be made once for all, and the still greater advantage of meeting scientific men in other departments. As science grows in details and in range, there is on the one hand an increased specialization, making it desirable for small groups of experts to meet together to discuss their special problems, while, on the other hand, almost every scientific question has ramifications extending to many sciences. Hence, the need of many separate societies and at the same time of a common meeting ground. When the American Association was organized, in 1848, its members could meet in one body; later they333 divided into two sections, one for the exact sciences and one for natural history. In 1882 nine sections were organized, but it was not until 1892 that botany was separated from zoölogy. At present the sections no longer suffice, and there must be either a further sub-division and a more efficient organization of the sections, or the American Association must become an administrative body, that will arrange for the simultaneous meetings of independent societies and the union of these societies in support of their common interests.
The obvious advantages of meeting together have now led nearly all the national scientific societies to select either the time of the American Association or Christmas week for joint meetings. It is unfortunate that they should be divided into two groups, and it must be admitted that neither midsummer nor the Christmas holidays are altogether suitable for the meetings. The American Association has this year made the experiment of selecting the end of June, immediately after the close of the college sessions, instead of a week in August. This has some advantages, but even at the beginning of the summer many men of science are either abroad or are engaged in scientific expeditions. The heat is apt to be excessive, interfering not only with the meetings, but also requiring some self-sacrifice on the part of scientific men when they leave their comfortable summer homes to travel through heat and dust to a hot and dusty city. Christmas week, divided by Sunday, is too short for a series of scientific meetings, especially for those who must travel from a distance. This led to the organization last winter of the Cordillerean Geological Society, the Western Society of Naturalists and the Western Philosophical Association. Local associations are, of course, valuable, but they should not interfere with one central meeting in the course of the year. The plan has been suggested of taking one week, either immediately after the New Year or in the early spring, for a general scientific gathering, which would include not only the exact and natural sciences, but also philology, history, etc. The plan would be to secure an adjournment of exercises or leave of absence in the case of universities, colleges, museums, Government departments, etc., with the understanding that it would be the duty of all those who were released from their regular work to attend the meetings.
The American Association last met in New York City in 1887, though there was a meeting in Brooklyn in 1894. The past thirteen and even the past six years have witnessed an extraordinary development in the educational and scientific institutions of the city. Columbia College and New York University have developed into great universities, each having found a new site and erected upon it buildings which might have been expected to come only as the growth of a century. The American Museum of Natural History has become one of the great museums of the world, millions of dollars having been spent on buildings. A botanical garden and a zoölogical park have been established, which promise to rival those of any of the European capitals. A well-equipped aquarium has been opened under the auspices of the city; the Metropolitan Museum of Art has been entirely rebuilt to accommodate its increasing collections; a magnificent building is in course of erection for the Public Library to contain its great assemblage of books, which with its endowment is largely the result of recent years. While Boston and Philadelphia have made great advances within the last few years, and Washington has become the chief scientific center of the United States, it is especially noteworthy that New York City has enjoyed an educational and scientific development commensurate with its material resources.
Jonas G. Clark, who ten years ago established at Worcester a university334 and christened it with his name, has died and left to the university several hundred thousand dollars, and on certain conditions practically the whole of his estate, which is said to be between five and ten million dollars. The will is a complicated document with numerous codicils, somewhat difficult to interpret and likely to give rise to legal complications. The history of Clark University has been curious and interesting. As in the case of the Johns Hopkins University, there was a difference of opinion between the founder and the president as to the scope of the institution. In both cases the founder had in view a more or less local college, while the president believed that we had colleges in sufficient number, but needed in the United States universities on German models, but going even further than Germany in making research rather than instruction the primary object of the institution. Johns Hopkins died very soon after the establishment of his university, and though there was for a while a good deal of difference of opinion in the board of trustees, the university idea triumphed. A college was, however, established in connection with it. At Clark University the founder lived for ten years, and appears to have altered several times his point of view. He withdrew his support, and the university work which began brilliantly was much reduced in range and quality. The greater part of the faculty removed in a body to the University of Chicago. It appears that at this time Mr. Clark bequeathed his money to the university only on condition that the president should resign, but later devised a compromise by which the university should continue as at present, while a partly independent college should be established in conjunction with it. The interpretation of the will, the value of the estate and the development of the university open problems that will only be settled in the course of time.
Europeans who look upon the United States as a material and commercial nation must find it difficult to interpret the great gifts that are continually made for the cause of higher education. Twenty-five years ago there were in America no universities in the sense in which the term is most properly employed. During this comparatively brief period the older institutions have become universities, and the great increase in expenditure has been met chiefly by voluntary contributions. The annual expenditure, for example, at Harvard and Columbia Universities is about a half million dollars beyond the tuition fees, and the money invested in grounds and buildings, is in the case of either university many millions. Then this period has witnessed the establishment of new universities, rivaling in endowment the older institutions. The Johns Hopkins University and Clark University have been mentioned above, but the most noteworthy instances are the University of Chicago, to which one benefactor still living has given eight million dollars, and Leland Stanford Junior University, the endowment of which reaches the enormous sum of thirty-five million dollars. At the same time, the State universities, directly supported by the people, are beginning to rival privately endowed institutions. It may be confidently asserted that no nation has ever so liberally supported higher education, and the wisdom of this liberality is now demonstrated, even from the most mercenary point of view, by the place the United States has taken in the world’s commerce. It will be still further demonstrated in the course of the next twenty-five years. It is possible that existing conditions are not favorable to literature and to art, but the future of science in the United States is assured beyond question.
It is sometimes said that Government control and individual initiative can not be united, but there is no justification for this view in the development of the educational and scientific institutions of the United States. Institutions335 established by private initiative have been assisted by the State, and State institutions have received large sums from private individuals. The New York institutions referred to above—the American Museum of Natural History, the Metropolitan Museum of Art, the Public Library, the Botanical Gardens and the Zoölogical Park—are in almost equal measure supported by the city and by citizens of the city. Johns Hopkins University, the University of Pennsylvania, Cornell University and other privately endowed institutions have received assistance from the State, without any decrease in private gifts, while the State universities, California for example, are receiving large private endowments in addition to their support from the State. These conditions may not last, but at all events they obtain at the present time, and we find the country in which the largest gifts from private individuals are made for education and science to be the country in which they are most liberally supported by the Government.
Never before has any government made such great appropriations for the development of the resources of the country or for the advance of science as the Congress which has just adjourned. We may take for example the Department of Agriculture, for which the appropriation is $4,023,500, an increase of more than $280,000 over the appropriation for the preceding year. Every one familiar with the conditions at Washington and throughout the country will know that this large sum of money is expended with the utmost economy, and there is no doubt but what the money invested by the nation is returned to the people many fold in the course of every year. Some of the items of the bill deserve special notice. Thus, a new agricultural experiment station is to be established in the Hawaiian Islands, and the work of the Weather Bureau is to be extended to them. The agricultural resources and capabilities of Porto Rico are to be investigated, and bulletins of information in English and in Spanish are to be distributed to the inhabitants. The division of chemistry is to investigate the use of food preservatives and coloring matter, determine their relations to health and establish the principles which should guide their use. The division of forestry receives an increase of $40,000 and the Weather Bureau an increase of over $35,000. Other items of the appropriation act are as follows: Biological Survey, $30,300, an increase of $2,740; Division of Botany, $43,080, an increase of $14,280; Nutrition Investigation, $17,500, an increase of $2,500; Division of Pomology, $18,400; Public Road Inquiry, $14,000, an increase of $6,000; Division of Statistics, $146,160; Library, $14,000; and Museum, $2,260.
While American men of wealth have given freely of their means for the promotion of education and science, they have not so often devoted their own time to its service. This is natural, as the wealth has in most cases been acquired by the present generation, and it is in succeeding generations, when families have been established, that leisure and wealth will give a class similar to that which has accomplished so much for Great Britain and to a lesser extent for Germany and France. Still, it is the case that the heads of two of our chief universities are men of great wealth, who have devoted not only their means, but also their services to the cause of education, and there are in our universities and other institutions many who hold their positions purely out of interest in their work, not as a means for their support. In the next generation there will probably be more representatives of a class to which belonged the Duke of Argyll, whose death we were compelled to record last month. Another man has since died of a somewhat similar type. When Colonel Lane-Fox somewhat unexpectedly succeeded to large estates in Wiltshire and Dorsetshire and assumed the336 name Pitt-Rivers, his chief interest seemed to be in the earth works and tumuli of Cranbourne Chase, and the extensive memoirs he has published and the museum he has established show what good use he made of the excavations. Some of the results of his earlier work will be found at Oxford, but he built at Farnham, in Dorsetshire, a museum which contains collections of the greatest possible value.
The communication in this issue signed ‘Physicist’ is worthy of note. If what its writer says is true, it is evident that a reputation as a brilliant inventor does not insure that its possessor is a safe writer about general physics. Our correspondent, who represents fairly the opinion of scientific men in general, finds fault with Mr. Tesla’s article in the June Century in many important particulars. During the years since Mr. Tesla’s notable invention of the polyphase alternate current transformer, he seems to have become less definite and exact in his thinking, and less productive as an inventor. The speculation and rhetoric of the Century article are certainly disappointing to every one who is trying to bring about an intelligent and sound view of science on the part of non-scientific people. Men of science everywhere should certainly make it their business to instruct people in general about the progress, and even the prospects, of science through the press, but it takes wisdom on the part of both writers and editors to know what is instructive and what is misleading. Honest criticism such as that of our correspondent is therefore highly desirable.
It is generally agreed that the most important advance of last year in the science of medicine was the discovery that the parasite causing malaria was transmitted from person to person by mosquitoes. Dr. Manson describes this discovery fully in this number of the Popular Science Monthly. This summer a crucial experiment is being made of a somewhat dramatic character. A mosquito-proof tent has been constructed, which is located in Italy, in the Campagna. In this Dr. Luigi Sambon, lecturer of the London Tropical School of Medicine, and Dr. G. C. Low will live until October, taking the utmost care not to be bitten by mosquitoes. If they escape malaria it will serve as corroborative evidence that the mosquito is the means of infection. On the other hand, several Englishmen, including Dr. Manson’s son, have offered themselves as subjects for the complementary experiment. They will live in a healthy district, but will definitely allow themselves to be bitten by mosquitoes which are known to be infected. These experiments will probably be particularly useful in demonstrating to the public at large the validity of the hypothesis derived last year from technical bacteriological evidence.
Punctuation, hyphenation, and spelling were made consistent when a predominant preference was found in this book; otherwise they were not changed. Inconsistent spacing in abbreviations was not changed.
Simple typographical errors were corrected; occasional unbalanced quotation marks retained.
Ambiguous hyphens at the ends of lines were retained.
Page 230: “spectrograph” was misprinted as “spectograph”; corrected here.
Page 315: “sporozooites” was printed that way.
Page 322: “uranium oxids” was printed that way.
Page 329: “‘Plant Geography of North American,’” may be a misprint for “America”.