
Title: Scientific American Supplement, No. 795, March 28, 1891
Author: Various
Release date: September 12, 2004 [eBook #13443]
Most recently updated: October 28, 2024
Language: English
Credits: Produced by Don Kretz, Juliet Sutherland, Victoria Woosley and the
Online Distributed Proofreading Team.
There will soon be inaugurated (probably about the 14th of July) a new establishment that has long been demanded by the laboring population, that is to say, a new labor exchange, the buildings of which, situated on Chateau d'Eau Street, are to succeed the provisional exchange installed in the vicinity of Le Louvre Street. The new structures have been erected from plans by Mr. Bouvard, and occupy an area of seventeen hundred meters.
The main work is now entirely terminated, but the interior decorations are not yet completely finished. The distribution comprises a vast meeting room, committee rooms for the various syndicates, offices in which the workmen of the various bodies of trades will find information and advice, and will be enabled to be put in relation with employers without passing through the more or less recommendable agencies to which they have hitherto been obliged to have recourse.
Upon the whole, the institution, if wisely conducted, is capable of bearing fruit and ought to do so, and the laboring population of Paris should be grateful to the municipal council for the six million francs that our ediles have so generously voted for making this interesting work a success. On seeing the precautions, perhaps necessary, that the laborer now takes against the capitalist, we cannot help instituting a comparison with the antique and solid organization of labor that formerly governed the trades unions. Each corporation possessed a syndic charged with watching over the management of affairs, and over the receipts and the use of the common resources. These syndics were appointed for two years, and had to make annually, at least, four visits to all the masters, in order to learn how the laborers were treated and paid, and how loyally the regulations of the corporation were observed. They rendered an account of this to the first assembly of the community and cited all the masters in fault.
Evidently, the new Labor Exchange will not cause a revival of these old ways of doing things (which perhaps may have had something of good in them), but we may hope that laborers will find in it protection against those who would require of them an excess of work, as well as against those who would preach idleness and revolt to them.—Le Monde Illustré.

NEW LABOR EXCHANGE—HALL FOR MEETINGS.
The controlling motive and direct purpose of the average newspaper are financial profit. One is now and then founded, and conducted even at a loss, to serve party, social, religious or other ends, but where the primary intent is unselfish there remains hope for monetary gain.
The first newspapers never dreamed of teaching or influencing men, but were made to collect news and entertainment and deal in them as in any other commodity. But because this was the work of intelligence upon intelligence, and because of conditions inherent in this kind of business, it soon took higher form and service, and came into responsibilities of which, in its origin, it had taken no thought. Wingate's "Views and Interviews on Journalism" gives the opinions of the leading editors and publishers of fifteen years ago upon this point of newspaper motive and work. The first notable utterance was by Mr. Whitelaw Reid, who said the idea and object of the modern daily newspaper are to collect and give news, with the promptest and best elucidation and discussion thereof, that is, the selling of these in the open market; primarily a "merchant of news." Substantially and distinctly the same ideas were given by William Cullen Bryant, Henry Watterson, Samuel Bowles, Charles A. Dana, Henry J. Raymond, Horace White, David G. Croly, Murat Halstead, Frederick Hudson, George William Curtis, E.L. Godkin, Manton Marble, Parke Godwin, George W. Smalley, James Gordon Bennett and Horace Greeley. The book is fat with discussion by these and other eminent newspaper men, as to the motives, methods and ethics of their profession, disclosing high ideals and genuine seeking of good for all the world, but the whole of it at last rests upon primary motives and controlling principles in nowise different or better or worse than those of the Produce Exchange and the dry goods district, of Wall Street and Broadway, so that, taking publications in the lump, it is neither untrue nor ungenerous, nor, when fully considered, is it surprising, to say that the world's doing, fact and fancy are collected, reported, discussed, scandalized, condemned, commended, supported and turned back upon the world as the publisher's merchandise.
The force and reach of this controlling motive elude the reckoning of the closest observation and ripest experience, but as somewhat measuring its strength and pervasiveness hear, and for a moment think, of these facts and figures.
The American Newspaper Directory for 1890, accepted as the standard compiler and analyst of newspaper statistics, gives as the number of regularly issued publications in the United States and territories, 17,760. Then when we know that these have an aggregate circulation for each separate issue—not for each week, or month, or for a year, but for each separate issue of each individual publication, a total of 41,524,000 copies—many of them repeating themselves each day, some each alternate day, some each third day and the remainder each week, month or quarter, and that in a single year they produce 3,481,610,000 copies, knowing, though dimly realizing, this tremendous output, we have some faint impression of the numerical strength of this mighty force which holds close relation to and bears strong influence upon life, thought and work, and which, measured by its units, is as the June leaves on the trees—in its vast aggregate almost inconceivable; a force expansive, aggressive, pervasive; going everywhere; stopping nowhere; ceasing never.
I am to speak to you of "The Business End" of the American newspaper; that is of the work of the publisher's department—not the editor's. At the outset I am confronted with divisions and subdivisions of the subject so many and so far reaching that right regard for time compels the merest generalization; but, as best I can, and as briefly as I can, I shall speak upon the topic under three general divisions:
First.—The personal and material forces which make the newspaper.
Second.—The sources of revenue from the joint working of these forces.
Third.—The direct office, bearing and influence of these forces.
It is but natural that the general public has limited idea of the personality and mechanism of the publication business, for much of its movement is at night, and there is separation and isolation of departments, as well as complicated relation of the several parts to the whole. Not many years ago a very few men and boys could edit, print and distribute the most important of newspapers, where now hundreds are necessary parts in a tremendous complexity. But even to-day, of the nearly 18,000 publications in the United States, more than 11,000 are of that class which, in all their departments, are operated by from two to four or five persons, and which furnish scant remuneration even for these. Among the thin populations and in the remote regions are thousands of weekly papers—and you may spell the weekly either with a double e or an ea— where there are two men and a boy, one of whom does a little writing and much scissoring, loafing among the corner groceries and worse, begging for subscribers, button-holing for advertisements, and occasionally and indiscriminatingly thrashing or being thrashed by the "esteemed contemporary" or the "outraged citizen;" the second of whom sets the type, reads the proofs, corrects them more or less, makes the rollers, works the old hand press, and curses the editor and the boy impartially; and the third of whom sweeps the office weekly, bi-weekly or monthly, inks the forms and sometimes pis them, carries the papers, and does generally the humble and diversified works of the "printer's devil," while between the three the whole thing periodically goes to the ---- level pretty sure to be reached now and then by papers of this class. Yet there are many of these country papers that Mr. Watterson once styled the "Rural Roosters" which are useful and honored, and which actively employ as editors and publishers men of fair culture and good common sense, with typographical and mechanical assistants who are worthy of their craft.
But the personal workers upon the great magazines and the daily newspapers are for each a battalion or a regiment, and in the aggregate a vast army. The Century Magazine regularly employs in its editorial department three editors and eight editorial assistants, of whom five are women; in the art department two artists in charge and four assistants, of whom three are women; in the business department fifty-eight persons, men and women—a total of seventy six persons employed on the magazine regularly and wholly, while the printers and binders engaged in preparing a monthly edition of 200,000 magazines are at least a duplicate of the number engaged in the editorial, art and business divisions.
The actual working force upon the average large daily newspaper, as well as an outline idea of the work done in each department, and of its unified result in the printed sheet, as such newspapers are operated in New York, Chicago and Boston, may be realized from an exhibit of the exact current status in the establishment of a well known Chicago paper.
In its editorial department there are the editor-in-chief, managing editors, city editors, telegraph editors, exchange editors, editorial writers, special writers and about thirty reporters—56 in all. Working in direct connection with this department, and as part of it, are three telegraph operators and nine artists, etchers, photographers and engravers; in the Washington office three staff correspondents, and in the Milwaukee office one such correspondent—making for what Mr. Bennett calls the intellectual end a force of 72 men, who are usually regarded by the business end as a necessary evil, to be fed and clothed, but on the whole as hardly worth the counting.
In the business and mechanical departments the men and women and their work are these:
The business office, for general clerical work, receiving and caring for advertisements, receiving and disbursing cash, and for the general bookkeeping, employs 24 men and women.
On the city circulation, stimulating and managing it within the city and the immediate vicinity, 10 persons.
On the country circulation, for handling all out-of-town subscriptions and orders of wholesale news agents, 30 persons.
On mailing and delivery, for sending out by mail and express of the outside circulation, and for distribution to city agents and newsboys, 31 persons.
In the New York office, caring for the paper's business throughout the East, the Canadas, Great Britain and Europe, two persons.
In the composing room, where the copy is put into type, and in the linotype room, where a part of the type-setting is done by machinery, 95 persons.
In the stereotype foundry, where the plates are cast (for the type itself never is put on the press), 11 persons.
In the press room, where the printing, folding, cutting, pasting and counting of the papers is done, 30 persons.
In the engine and dynamo room, 8 persons.
In the care of the building, 3 persons.
These numbers include only the minimum and always necessary force, and make an aggregate of 316 persons daily and nightly engaged for their entire working time, and borne on a pay roll of six thousand dollars a week for salaries and wages alone.
But this takes no account of special correspondents subject to instant call in several hundred places throughout the country; of European correspondents; of 1,900 news agents throughout the West; of 200 city carriers; of 42 wholesale city dealers, with their horses and wagons; of 200 branch advertisement offices throughout the city, all connected with the main office by telephone; and of more than 3 000 news boy—ash;all making their living, in whole or in part, from work upon or business relations with this one paper—a little army of 6,300 men, women, and children, producing and distributing but one of the 1,626 daily newspapers in the United States.
The leading material forces in newspaper production are type, paper, and presses.
Printing types are cast from a composition which is made one-half of lead, one-fourth of tin, and one-fourth of antimony, though these proportions are slightly reduced, so as to admit what the chemist calls of copper "a trace," the sum of these parts aiming at a metal which "shall be hard, yet not brittle; ductile, yet tough; flowing freely, yet hardening quickly." Body type, that is, those classes ever seen in ordinary print, aside from display and fancy styles, is in thirteen classes, the smallest technically called brilliant and the largest great primer.
In the reading columns of newspapers but four classes are ordinarily used—agate for the small advertisements; agate, nonpareil, and minion for news, miscellany, etc., and minion and brevier for editorials—the minion being used for what are called minor editorials, and the brevier for leading articles, as to which it may be said that young editorial writers consider life very real and very earnest until they are promoted from minion to brevier.
A complete assortment of any one of these classes is called a font, the average weight of which is about 800 pounds. Whereas our alphabet has 26 letters, the compositor must really use of letters, spaces, accent marks, and other characters in an English font 152 distinct types, and in each font there are 195,000 individual pieces. The largest number of letters in a font belongs to small e—12,000; and the least number to the z—200. The letters, characters, spaces, etc., are distributed by the printer in a pair of cases, the upper one for capitals, small capitals, and various characters, having 98 boxes, and the lower one, for the small letters, punctuation marks, etc., having 54 boxes.
A few newspapers are using typesetting machines for all or part of their composition. The New York Tribune is using the Linotype machine for all its typesetting except the displayed advertisements, and other papers are using it for a portion of their work, while still others are using the Rogers and various machines, of which there are already six or more. It seems probable that within the early future newspaper composition will very generally be done by machinery.
It has been suggested to me that many of my hearers this evening know little or nothing of the processes of the printer's art, and that some exposition of it may interest a considerable portion of this audience.
The vast number of these little "messengers of thought" which are required in a single modern daily newspaper is little known to newspaper readers. Set in the manner of ordinary reading, a column of the New York Tribune contains 12,200 pieces, counting head lines, leads, and so on; while, if set solidly in its medium-sized type, there are 18,800 pieces in one column, or about 113,000 in a page, or about 1,354,000 in one of its ordinary 12-page issues. A 32-page Sunday issue of the New York Herald contains nearly, if not quite, 2,500,000 distinct types and other pieces of metal, each of which must be separately handled between thumb and finger twice—once put into the case and once taken out of it—each issue of the paper. No one inexperienced in this delicate work has the slightest conception of the intensity of attention, fixity of eye, deftness of touch, readiness of intelligence, exhaustion of vitality, and destruction of brain and nerve which enters into the daily newspaper from type-setters alone.
Each type is marked upon one side by slight nicks, by sight and touch of which the compositor is guided in rapidly placing them right side up in the line. They are taken, one by one, between thumb and forefinger, while the mind not only spells out each word, but is always carrying phrases and whole sentences ahead of the fingers, and each letter, syllable and word is set in its order in lines in the composing stick, each line being spaced out in the stick so as to exactly fit the column width, this process being repeated until the stick is full. Then the stickful is emptied upon a galley. Then, when the page or the paper is "up," as the printers phrase it, the galleys are collected, and the foreman makes up the pages, article by article, as they come to us in the printed paper—the preliminary processes of printing proofs from the galleys, reading them by the proof readers, who mark the errors, and making the corrections by the compositors (each one correcting his own work), having been quietly and swiftly going on all the while. The page is made up on a portable slab of iron, upon which it is sent to the stereotyping room. There wet stereotyping paper, several sheets in thickness, is laid over the page, and this almost pulpy paper is rapidly and dexterously beaten evenly all over with stiff hair brushes until the soft paper is pressed down into all the interstices between the type; then this is covered with blankets and the whole is placed upon a steam chest, where it is subjected to heat and pressure until the wet paper becomes perfectly dry. Then, this dried and hardened paper, called a matrix, is placed in a circular mould, and melted stereotype metal is poured in and cooled, resulting in the circular plate, which is rapidly carried to the press room, clamped upon its cylinder, and when all the cylinders are filled, page by page in proper sequence, the pressman gives the signal, the burr and whirr begin, and men and scarcely less sentient machines enter upon their swift race for the early trains. As a matter of general interest it may be remarked that this whole process of stereotyping a page, from the time the type leaves the composing room until the plate is clamped upon the press, averages fifteen minutes, and that cases are upon record when the complex task has been accomplished in eleven minutes.
The paper is brought from the mill tightly rolled upon wooden or iron cores. Some presses take paper the narrow way of the paper, rolls for which average between 600 and 700 pounds. Others work upon paper of double the width of two pages, that is, four pages wide, and then the rolls are sometimes as wide as six feet, and have an average weight of 1,350 pounds. Each roll from which the New York Tribune is printed contains an unbroken sheet 23,000 feet (4-1/3 miles) long. A few hours before the paper is to be printed, an iron shaft having journal ends is passed through the core, the roll is placed in a frame where it may revolve, the end of the sheet is grasped by steel fingers and the roll is unwound at a speed of from 13 to 15 miles an hour, while a fan-like spray of water plays evenly across its width, so that the entire sheet is unrolled, dampened, for the better taking of the impression to be made upon it, and firmly rewound, all in twenty minutes. Each of these rolls will make about 7,600 copies of the Tribune.
When all is ready, paper and stereotyped pages in place, and all adjustments carefully attended to, the almost thinking machine starts at the pressman's touch, and with well nigh incredible speed prints, places sheet within sheet, pastes the parts together, cuts, folds and counts out the completed papers with an accuracy and constancy beyond the power of human eye and hand.
The printing press has held its own in the rapid advance of that wonderful evolution which, within the last half century, in every phase of thought and in every movement of material forces placed under the dominion of men, has almost made one of our years the equivalent of one of the old centuries. Within average recollection the single cylinder printing machine, run by hand or steam, and able under best conditions to print one side of a thousand sheets in one hour, was the marvel of mankind. In 1850, one such, that we started in an eastern Ohio town, drew such crowds of wondering on-lookers that we were obliged to bar the open doorway to keep them at a distance which would allow the astonishing thing to work at all.
To-day, in the United States alone, five millions of dollars are invested in the building of printing presses, many of which, by slightest violence to figure of speech, do think and speak. Inspiration was not wholly a thing of long-gone ages, for if ever men received into brain and worked out through hand the divine touch, then were Hoe, and Scott, and Campbell taught of God.
Under existing conditions newspapers of any importance, in the smaller cities, use one and sometimes two presses, capable of producing from 7,000 to 9,000 complete eight page papers each hour, each machine costing from $10,000 to $15,000. Papers of the second class in the large cities use treble or quadruple this press capacity, while the great papers, in the four or five leading cities, have machinery plants of from four to ten presses of greatest capacity, costing from $32,000 to $50,000 each, and able to produce papers of the different numbers of pages required, at a speed of from 24,000 to 90,000 four page sheets, or of from 24,000 to 48,000 eight, ten, or twelve page sheets per hour, each paper complete as you receive it at your breakfast table—printed, pasted, cut and folded, and the entire product for the day accurately counted in lots of tens, fifties, hundreds or thousands, as may be required for instantaneous delivery, while, as if to illustrate and emphasize the ever upward trend of public demand for the day's news, quick and inclusive, Hoe & Co. are now building machines capable of producing in all completeness 150,000 four page papers each hour.
All this tremendous combination of brain, nerve, muscle, material, machinery and capital depends for its movement and remuneration upon but two sources of income—circulation and advertisements—the unit measurements of which are infinitesimal—for the most part represented by wholesale prices; from one-half a cent to two cents per copy for the daily newspaper, and in like proportion for the weeklies and monthlies; and by from one-tenth of a cent to one cent per line per thousand of circulation for advertising space. Verily, in a certain and large sense, the vast publishing interests rest upon drops of water and grains of sand. Under right conditions no kind of business or property is more valuable, and yet no basis of values is more intangible. Nothing in all trade or commerce is so difficult to establish or more environed by competitions, and yet, once established, almost nothing save interior dry rot can pull it down. It depends upon the judgment and favor of the million, yet instances are few where any external force has seriously and permanently impaired it.
About two hundred years have gone since the publication of the first number of the first American newspaper. It was a monthly, called Publick Occurrences, both Foreign and Domestic, first printed September 25, 1690, by Richard Pierce, and founded by Benjamin Harris. At that time public favor did seem to control newspaper interests, for that first paper aroused antagonism, and it was almost immediately suppressed by the authorities. Only one copy of it is now in existence, and that is in London. The first newspaper to live, in this country, was the Boston News Letter, first issued in 1704 and continued until 1776. New York's first newspaper, the New York Gazette, appeared October 16, 1725. At the outbreak of the revolution there were 37 newspapers, and in 1800 there were 200, of which several were dailies. In 1890 there were 17,760, of which there were 13,164 weeklies, 2,191 monthlies, 1,626 dailies, 280 semi-monthlies, 217 semi-weeklies, 126 quarterlies, 82 bi-weeklies, 38 bi-monthlies, and 36 tri-weeklies.
The circulations belong largely to the weeklies, monthlies and dailies, the weeklies having 23,228,750, the monthlies 9,245,750, the dailies 6,653,250, leaving only 2,400,000 for all the others.
The largest definitely ascertainable daily average circulation for one year, in this country, has been 222,745. Only one other daily paper in the world has had more—Le Petit Journal, in Paris, which really, as we understand it, is not a newspaper, but which regularly prints and sells for one sou more than 750,000 copies. The largest American weekly is the Youth's Companion, Boston, 461,470. The largest monthly is the Ladies' Home Journal, Philadelphia, 542,000. The largest among the better known magazines is the Century, 200,000. Of the daily papers which directly interest us—those of the city of New York—the actual or approximate daily averages of the morning papers are given by "Dauchy's Newspaper Catalogue" for 1891, as follows: Tribune, daily, 80,000; Sunday, 85,000. Times, daily, 40,000; Sunday, 55,000. Herald, daily, 100,000; Sunday, 120,000. Morning Journal, 200,000. Press, daily, 85,000; Sunday, 45,000. Sun, daily, 90,000; Sunday, 120,000. World, daily, 182,000; Sunday, 275,000. Of the afternoon papers, Commercial Advertiser, 15,000; Evening Post, 18,000; Telegram, 25,000; Graphic (not the old, but a new one), 10,000; Mail and Express, 40,000; News, 173,000; Evening Sun, 50,000; Evening World, 168,000. The entire circulation of New York dailies, including with those named others of minor importance, and the German, French, Italian, Bohemian, Hebrew and Spanish daily newspapers, is 1,540,200 copies.
Obviously, there is and must be ceaseless, incisive and merciless competition in securing and holding circulations, as well as in the outward statements made of individual circulations to those who purchase advertising space. In this, as in all other forms of enterprise, there are honest, clean-cut and business-like methods, and there are the methods of the time-server, the trickster and the liar.
The vastly greater number of publications secure and hold their clientage by making the best possible goods, pushing them upon public patronage by aggressive and business-like means, and selling at the lowest price consistent with excellence of product and fairness alike to producer and consumer. But of the baser sort there are always enough to make rugged paths for those who walk uprightly, and to contribute to instability of values on the one hand, and on the other to flooding the country with publications which the home and the world would be better without. Every great city has more of the rightly made and rightly sold papers than of the other sort, and the business man, the working man, the professional man, the family, no matter of what taste, or political faith, or economic bias, or social status, or financial plenty or paucity, can have the daily visits of newspapers which are able, brilliant, comprehensive, clean and honest. But all the time, these men and families will have pressed upon their attention and patronage, by every device and artifice of the energetic and more or less unscrupulous publisher, other papers equally able and brilliant and comprehensive, but bringing also their burden of needless sensationalism and mendacity, undue expansion of all manner of scandal, amplification of every detail and kind of crime, and every phase of covert innuendo or open attack upon official doing and private character—the whole infernal mass procured, and stimulated and broadcast among the people by the "business end of it," with the one and only intent of securing and holding circulation.
Take a representative and pertinent example. Eight years ago there were in New York ten or eleven standard newspapers, as ably and inclusively edited and as energetically and successfully conducted, business-wise, as they are now. Even at their worst they were decently mindful of life's proprieties and moralities and they throve by legitimate sale of the most and best news and the best possible elucidation and discussion thereof. The father could bring the paper of his choice to his breakfast table with no fear that his own moral sense and self-respect might be outraged, or that the face of his wife might be crimsoned and the minds of his children befouled. But there came from out of the West new men and new forces, quick to see the larger opportunity opened in the very center of five millions of people, and almost in a night came the metamorphosis of the old World into the new. It was deftly given out that existing conditions were inadequate to the better deserts of the Knickerbocker, the Jersey-man, and the Yankee, and that a new purveyor of more highly seasoned news and a more doughty champion of their rights and interests was hither from the land of life and movement—at two cents per copy. There was a panic in New York newspaper counting rooms, and prices tumbled in two days from the three and four cents of fair profit to the two and three cents of bare cost or less. The new factors in demoralization cared nothing for competition in prices or legitimate goods, for they had other ideas of coddling the dear people. Ready to their purpose lay disintegrated Liberty, waiting for a rock upon which to plant her feet and raise her torch, and the new combination between the world, the flesh and the devil, waiting and ready for access to the pockets of the public, was only too ready to set up Liberty and itself at one stroke, if only the joint operation could be done without expense to itself. The people said, "What wonderful enterprise!" "What a generous spirit!" The combination, with tongue in cheek and finger laid alongside nose, said to itself as it saw its circulation spring in one bound from five figures into six, "Verily we've got there! for these on the Hudson are greater gudgeons than are they on the Mississippi." From then until now, with an outward semblance and constant pretense of serving the people; with blare of trumpet and rattle of drum; with finding Stanley, who never had been lost; with scurrying peripatetic petticoats around the globe; with all manner of unprofessional and illegitimate devices; with so-called "contests" and with all manner of "schemes" without limit in number, kind, or degree; with every cunningly devised form of appeal to curiosity and cupidity —from then until now that combination has been struggling to hold and has held an audience of the undiscriminating and the unthinking. But, further, and worse, a short-sighted instinct of self-preservation has led other papers to follow somewhat at a distance in this demoralizing race. None of them has gone to such lengths, but the tendency to literary, mental and moral dissipation induced by a hitherto unknown form of competition has swerved and largely recast the methods of every New York daily save only the Tribune, Times, Commercial Advertiser, and Evening Post, while the converse side of securing business clientage is illustrated in a way that would be amusing if it were not pathetic, by that abnormal and fantastic cross between news and pietetics which mails and expresses itself to the truly good. These are forms of competition which the business end of legitimately conducted newspapers is compelled to meet. In a certain way these methods do succeed, but how, and how long and how much shall they succeed except by unsettling the mental and moral poise of the people, and by setting a new and false pace for publishers everywhere whose thoughts take less account of means than of ends? Which shall we hold in higher esteem and in our business patronage—Manton Marble and Hurlbut, gentlemen, scholarly, wise leaders, conscientious teachers, with barely living financial income; or their successors, parvenus, superficial, meretricious, false guides, time-serving leaders, a thousand dollars a day of clear profit, housed in the tower of Babel?
Considered in the large, the circulation side of the American newspaper has many indefensible aspects. As "nothing succeeds like success," or the appearance of success, the prestige of not a few newspapers is ministered unto by rotund and deceptive representations of circulation. Then, as few can live, much less profit, on their circulations alone, it becomes greatly important to make the advertiser see circulations through the large end of the telescope, and so the fine art of telling truth without lying is a live and perennial study in many counting rooms. Discussing the circulation question not long ago with the head of a leading religious paper, he told me that the number of copies he printed was a thing that he never stated definitely, because the publishers of the other religious newspapers lied so about their circulations that he would do himself injustice if he were to tell the truth about his own. The secular papers should set an example for their religious brethren, but they do not, for from many of them there is lying—systematic, persistent, and more or less colossal. Not long ago, within a few days of each other, three men who were simultaneously employed on a certain paper told me their actual circulation, confidentially, too. One of them put it at 85,000, the second at 110,000, and the third at 130,000, and each of them lied, for their lying was so diversified and entertaining that I felt a real interest in securing the truth, and so I took some trouble to ask the pressman about it. He told me, very confidentially, that it was 120,000—and he lied.
By this time my interest was so heightened that I told my personal friend, the publisher, about the inartistic and incoherent mendacity of his subordinates, whereupon he laughingly showed me his circulation book, which clearly, and I have no doubt truthfully, exhibited an average of 88,000. The wicked partner is nearly always ready to show the actual record of the counting machines on the presses, and "figures never lie" but the truth-telling machines which record actual work of the impression cylinders make no mention of damaged copies thrown aside, of sample copies, files, exchanges, copies kept against possible future need, copies unsold, copies nominally sold but sooner or later returned and finally sold to the junk shop, and all that sort of thing. One prints a large extra issue on a certain day for some business corporation which has its own purpose to serve by publication of an article in its own interest, whereby many thousands of copies are added to that day's normal output, and he makes the exceptional number for that day serve as the exponent of his circulation until good fortune brings him a similar and possibly larger order, and his circulation is reported as "still increasing." Another struck a "high-water mark" of "190,500" the day after Mr. Cleveland was elected, and that has been the implied measure of circulation for the last six years. Another, during a heated political campaign, or a great financial crisis, or some other dominant factor in public interest, makes a large and genuine temporary increase, but the highest mark gained does enforced duty in the eyes of the marines until another flood tide sweeps him to a greater transient height. These are types of the competitions of the circulation liar. At this very hour there are four daily newspapers each of which has the largest circulation in the United States. Of the nearly 18,000 American publications only 103 furnish detailed, open, and entirely trustworthy statements of circulation.
As to the general public this is no great matter, but to the vast number of business men who buy the real or fancied publicity afforded by newspaper advertising it is of exceeding importance. That the large buyers of advertising space are not more and oftener swindled is because they understand the circulation extravaganza and buy space according to their understanding. The time is coming, and it should come soon, when newspaper circulations shall be open to the same inspection and publicity as is now the case with banks and insurance companies, and when the circulation liar and swindler shall be amenable to the same law and liable to the same penalty as stands against and is visited upon any other perjurer and thief.
(To be continued.)
In the May (1890) number of the Therapeutic Gazette I furnished some contribution to the "Treatment of Hay Fever." I reported therein a favorable result in the treatment of this mysterious disease in the experience of my last year's cases.
My experience of this year is far more gratifying, and worthy of receiving a wide publicity.
I treated six cases in all, four of which have been habitual for years to hay fever proper without complications, while the other two used to have the disease aggravated with reflex asthma and bronchial catarrh. I succeeded in preventing the outbreak of the disease in every individual case. The treatment I applied was very simple, and consisted of the following:
From the fact that I had known all my patients from previous years, I ordered them to my office two weeks before the usual onset of the disease. I advised them to irrigate the nose with a warm solution of chloride of sodium four times a day—morning, noon, evening, and on retiring; and, a few minutes after the cleansing of the parts, had the nares thoroughly sprayed with peroxide of hydrogen and c.p. glycerin, half and half. Those subject to a conjunctivitis I prescribed a two per cent. solution of boric acid as a wash. At this period no internal medication was given, but three days previous to the usual onset of the disease I prescribed phenacetin and salol, five grains of each three times a day.
On the respective expected days, to the great surprise of all the members concerned successively, who have been in the habit of getting the disease almost invariably at a certain date, no hay fever symptoms appeared, though everyone had been the victim of the disease for a great number of years, varying from five to nineteen years' standing.
It is self-understood that this treatment was kept up all through the season, and, as no symptoms developed, the applications were reduced, toward the termination, to twice and once a day. The internal medication, however, was stopped after the expiration of the first week, and all the patients could attend to their various respective vocations, something they never have been able to do in previous years.
In two cases, though no nasal symptoms developed, about two weeks after the calculated onset, slight symptoms of asthma made their appearance. However, I could easily suppress them at this time with the aid of the hand atomizer and ozonizer, a very ingenious little apparatus, of which I gave a thorough description in my last year's article. I used the ozone inhalations every four hours, in connection with the internal administration of the following prescription:
Rx Iodide of ammonia, 8;
Fl. ex. quebracho, 30;
Fl. ex. grindelia robusta, 15;
Tr. lobelia, 12;
Tr. belladonna, 8;
Syr. pruni, virg., q.s., ad 120.
Sig.—Teaspoonful three or more times during twenty-four
hours.
However, toward the end of the fourth week, especially in one case—a stout, heavy-set gentleman—very grave asthmatic symptoms developed, which compelled me to apply Chapman's spinal ice bag, as well as resort to the internal administration of large doses of codeine during the paroxysm, with the most beneficial result.
I gave also oxygen inhalations a fair trial in the two cases. I find them to act very soothingly in the simple asthma, facilitating respiration after a few minutes; but during the paroxysmal stage they cannot be utilized, for the reason that respiration is short and rapid, and does not permit of a control in the quantity of the gas to be inhaled. Consequently, it is either of little use as a remedy; or, if too much is taken, a disagreeable headache will be the consequence.
During the catarrhal stage, which, however, was very mild compared with last year, I derived great benefit from the administration of codeine, in combination with terpine hydrate, in the pill form. The codeine has the advantage over all other opium preparations that it does not affect the digestive organs, and still acts in a soothing manner. While during last year's sickness my patients lost from ten to twenty pounds of their bodily weight, this year but one lost eight pounds and the other five pounds.
As the etiology of this troublesome disease is yet enveloped in obscurity, we may fairly conclude, by the success of my treatment, if it should meet with the confirmation of the profession, that the much pretended sensitive area, situated, according to Dr. Sajous, "at the posterior end of the inferior turbinated bones and the corresponding portion of the septum," or, according to Dr. John Mackenzie, who locates this area "at the anterior extremity of the inferior turbinated bone," need not necessarily be removed or destroyed by cautery, in order to accomplish a cure of hay fever proper.
I examined my patients twice a week, and the closest rhinoscopical exploration would not reveal the slightest pathological change in the mucous membrane of the nares.
Now, what is the etiological factor of the disease? Is it a specific germ conveyed by the air to the parts and—locus minoris resistencia—deposited at the pretended area, or is the germinal matter present in the nasal mucous membrane with certain persons, and requires only at a certain time and under certain conditions physiological stimulation to manifest periodical pathological changes, which give rise to the train of symptoms called hay fever? Dropping all hypothetical reasoning, I think some outside vegetable germ is causing the disease in those predisposed, and peroxide of hydrogen acts on them as it does on the pus corpuscles, i.e., drives them out when and wherever it finds them. I hope the profession will give this new measure a thorough trial and report their results.—Therapeutic Gazette.
In the Kew Bulletin for January an interesting account is given of the identification of the plant yielding the rhizome employed to make the well-known Chinese preserved ginger. As long ago as 1878 Dr. E. Percival Wright, of Trinity College, Dublin, called the attention of Mr. Thiselton Dyer to the fact that the preserved ginger has very much larger rhizomes than Zingiber officinale, and that it was quite improbable that it was the product of that plant. The difficulty in identifying the plant arose from the fact that, like many others cultivated for the root or tuber, it rarely flowers. The first flowering plant was sent to Kew from Jamaica by Mr. Harris, the superintendent of the Hope Garden there. During the past year the plant has flowered both at Dominica in the West Indies and in the Botanic Garden at Hong-Kong. Mr. C. Ford, the director of the Botanic Garden at Hong-Kong, has identified the plant as Alpinia Galanga, the source of the greater or Java galangal root of commerce. Mr. Watson, of Kew, appears to have been the first to suggest that the Chinese ginger plant is probably a species of Alpinia, and possibly identical with the Siam ginger plant, which was described by Sir J. Hooker in the Botanical Magazine (tab. 6,946) in 1887 as a new species under the name of Alpinia zingiberina. Mr. J.G. Baker, in working up the Scitamineæ for the "Flora of British India," arrived at the conclusion that it is not distinct from the Alpinia Galanga, Willd. The Siam and Chinese gingers are therefore identical, and both are the produce of Alpinia Galanga, Willd.
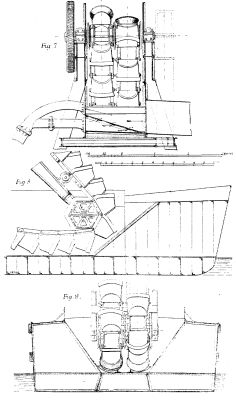
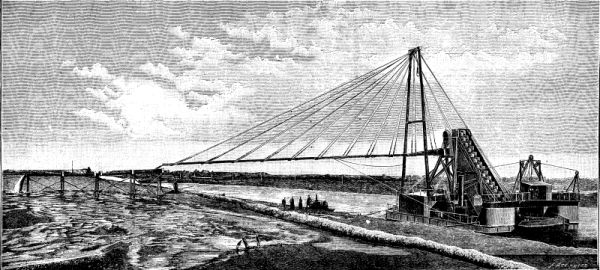
We illustrate a floating elevator and spoil distributor constructed by Mr. A.F. Smulders, Utrecht, Holland, for removing dredged material out of barges at the Baltic Sea Canal Works. We give a perspective view showing the apparatus at work, and on a page plate are given plans, longitudinal and cross sections, with details which are from Engineering. The dredged material is raised out of the launches or barges by means of a double ranged bucket chain to a height of 10.5 meters (34 ft. 5 in.) above the water line, from whence it is pushed to the place of deposition by a heavy stream of water supplied by centrifugal pumps.
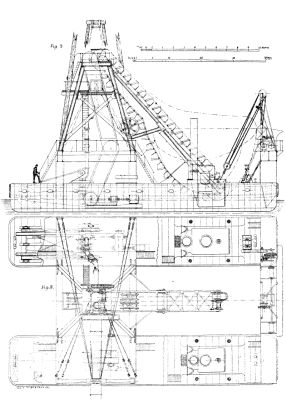
FLOATING ELEVATOR AND SPOIL DISTRIBUTOR FOR THE BALTIC SEA CANAL.--FIGS. 2, 3
The necessary machinery and superstructure are supported on two vessels connected, as shown in Figs. 4 and 5, with cross girders, a sufficient width being left between each vessel to form a well large enough for a barge to float into, and for the working of the bucket ladder utilized in raising the material from the barges. The girders are braced together and carry the framing for the bucket chains, gears, etc.
The port vessel is provided with a compound engine of 150 indicated horse power, with injection condenser actuating two powerful centrifugal pumps, raising water which enters by a series of holes into the bottom of the shoots underneath the dredged material, carrying the material to the conduit (as indicated on Fig. 4 and in detail on Figs. 6 and 7).
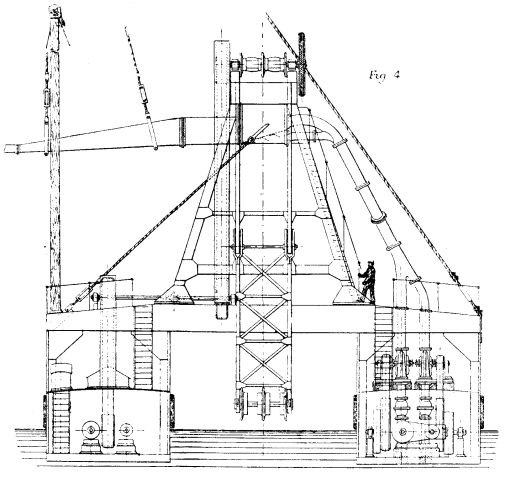
FLOATING ELEVATOR AND SPOIL DISTRIBUTOR FOR THE BALTIC SEA CANAL.--FIGS. 4.
A steel boiler of 80 square meters (860 square feet) heating surface, and 6 atmospheres (90 lb.) working pressure, supplies steam to the engine. Forward on the deck of the same vessel there is a vertical two-cylinder high pressure engine of 30 indicated horse power, which helps to bring the barge to the desired position between the parallel vessels. A horizontal two-cylinder engine of the same power, fitted with reversing gear, placed in the middle of the foremost iron girder, raises and lowers the bucket ladder by the interposition of a strongly framed capstan, as shown on Fig. 5. The gearing throughout is of friction pulleys and worm and wormwheel. It is driven by belts.
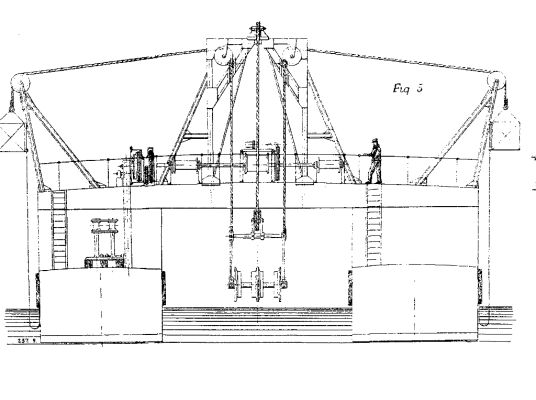
FLOATING ELEVATOR AND SPOIL DISTRIBUTOR FOR THE BALTIC SEA CANAL.--FIG. 5.
In the starboard vessel there is a compound engine of 100 indicated horse power, with injection condenser, working the bucket chain by means of belts and wheel gearing, as shown on Fig. 2. A marine boiler of 46 square meters (495 square feet) heating surface and 6 atmospheres (90 lb.) working pressure, supplies steam. In this vessel, it may be added, there is a cabin for the crew.
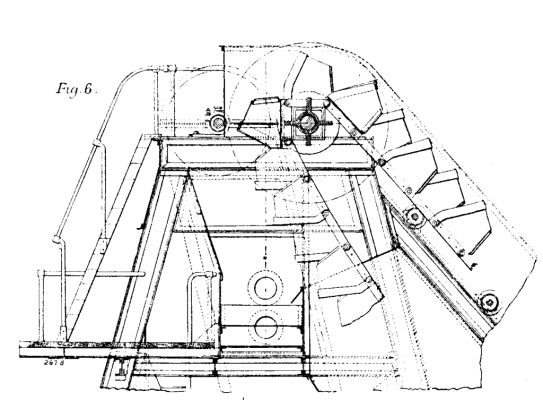
FLOATING ELEVATOR AND SPOIL DISTRIBUTOR FOR THE BALTIC SEA CANAL.--FIG. 6.
The dimensions of the vessels are as follows; Extreme length, 25 meters (82 ft.); breadth, 4.5 meters (14 ft. 9 in.); depth (moulded), 2.7 meters (6 ft. 6¾ in.); average draught of water, 1.4 meters (4 ft. 7 in.); space between the ships, 6.55 meters (21 ft. 6 in.) The iron structure connecting the ships is composed of four upright box-form stanchions on both ships, connected at the top by two strong box girders with tie pieces supporting the main framing. This main framing, also of the "box girder" form, is strengthened with angle irons and braced together at the tops by a platform supporting the gearing of the bucket chains, as shown on Fig. 5. The buckets have a capacity of 160 liters (5.65 cubic feet) and the speed in travel is at the rate of 25 to 30 buckets per minute, so that with both ladders working, 50 to 60 buckets are discharged per minute. The top tumbler shaft is placed at a height of 13 meters (42 ft. 8 in.) above the water line (Fig. 4), and the dredge conduit has a length of 50 meters (164 ft.), Fig. 1. The shooting is done at a height of 8.5 meters (27 ft. 10 in.) above the water line, and the shoot catches the dredged products at a height of 10.5 meters (34 ft. 5 in.) above the water line, the sliding gradient being 4 to 100. The dredge conduit is carried by timberwork resting on two of the upright box form stanchions.
FLOATING ELEVATOR AND SPOIL DISTRIBUTOR FOR THE BALTIC SEA CANAL.--FIGS. 7,8,9.
All cables are of galvanized steel and provided with open twin buckles. The main parts of the apparatus are of steel, and all pieces subject to wear and tear are fitted with bushes so formed that they can be easily replaced.
IMPROVED FLOATING ELEVATOR AND SPOIL DISTRIBUTOR.
The quantity of suitable soil removed by these apparatus amounts to 350 cubic meters (12,360 square feet) per hour. Four plants of similar construction have been built for the new Baltic Sea Canal, besides a fixed elevator of the same power and disposition, with the exception that the top tumbler shaft was suspended at a height of 16.1 meters (51 ft. 10 in.) above the water line, and the dredge conduit placed at a distance of 13 meters (43 ft.) from it.
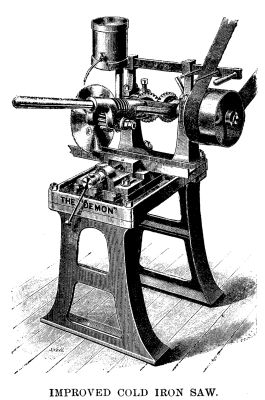 The engraving given herewith shows a general view
of the "Demon" cold saw, designed for cutting iron,
mild steel, or other metals of fairly large sections, that
is, up square or round, and any rectangular section up
to 8 in. by 4 in. The maker, Mr. R.G. Fiege, of London,
claims for this appliance that it is a cold iron saw,
at once powerful, simple and effective. It is always in
readiness for work, can be worked by inexperienced
workmen. The bed plate has T slots, to receive a parallel
vise, which can be fixed at any angle for angular
cutting. The articulated lever carries a saw of 10 in.
or 12 in. diameter, on the spindle of which a bronze
pinion is fixed, gearing with the worm shown. The
latter derives motion from a pair of bevel wheels,
which are in turn actuated from the pulley shown in
the engraving. The lever and the saw connected with
it can be raised and held up by a pawl while the work
is being fixed. In small work the weight of the lever
itself is found sufficient to feed the saw, but in heavier
work it is found necessary to attach a weight on the
end of the lever. The machine is fitted with fast and
loose pulleys, strap fork and bar. We are informed
that one of these machines is capable of making 400
cuts through bars of Bessemer steel 4 in. diameter,
each cutting occupying six minutes on an average,
without changing the saw.—Industries.
The engraving given herewith shows a general view
of the "Demon" cold saw, designed for cutting iron,
mild steel, or other metals of fairly large sections, that
is, up square or round, and any rectangular section up
to 8 in. by 4 in. The maker, Mr. R.G. Fiege, of London,
claims for this appliance that it is a cold iron saw,
at once powerful, simple and effective. It is always in
readiness for work, can be worked by inexperienced
workmen. The bed plate has T slots, to receive a parallel
vise, which can be fixed at any angle for angular
cutting. The articulated lever carries a saw of 10 in.
or 12 in. diameter, on the spindle of which a bronze
pinion is fixed, gearing with the worm shown. The
latter derives motion from a pair of bevel wheels,
which are in turn actuated from the pulley shown in
the engraving. The lever and the saw connected with
it can be raised and held up by a pawl while the work
is being fixed. In small work the weight of the lever
itself is found sufficient to feed the saw, but in heavier
work it is found necessary to attach a weight on the
end of the lever. The machine is fitted with fast and
loose pulleys, strap fork and bar. We are informed
that one of these machines is capable of making 400
cuts through bars of Bessemer steel 4 in. diameter,
each cutting occupying six minutes on an average,
without changing the saw.—Industries.
The railway system of the Argentine Republic is separated from the Chilian system by the chain of the Andes. The English contractors, Messrs. Clark & Co., have undertaken to connect them by a line which starts from Mendoza, the terminus of the Argentine system, and ends at Santa Rosa in Chili, with a total length of 144 miles. The distance from Buenos Ayres to Valparaiso will thus be reduced to 816 miles. The Argentine lines are of 5.4 foot gauge, and those of Chili of 4.6 foot.
The line in course of construction traverses an extremely hilly region. The starting and terminal points are at the levels of 2,338 feet (Mendoza) and 2,706 feet (Santa Rosa) above the sea; the lowest neck of the chain is at the level of 11,287 feet.
Study having shown that a direction line without tunnels, and even with the steepest gradients for traction by adhesion, would lead to a considerable lengthening of the line, and would expose it to avalanches and to obstructions by snow, there was adopted upon a certain length a rack track of the Abt system, with gradients of 8 per cent., and the neck is traversed by a tunnel 3 miles in length and 1,968 feet beneath the surface. The number and length of the tunnels upon the two declivities, moreover, are considerable. They are all provided with rack tracks. The first 80 miles, starting from Mendoza, are exploited by adhesion, with maximum gradients of 2½ per cent. Upon the remaining 64 miles, traction can be effected either by adhesion or racks.
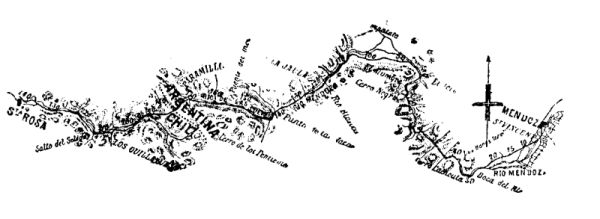
FIG. 1.—REGION TRAVERSED BY THE RAILWAY THROUGH THE ANDES.
The track is of 3.28 foot gauge, and this will necessitate trans-shipments upon the two systems. The rails weigh 19 pounds to the running foot in the parts where the exploitation can be effected either through adhesion or racks, and 17 pounds in those in which adhesion alone will be employed.
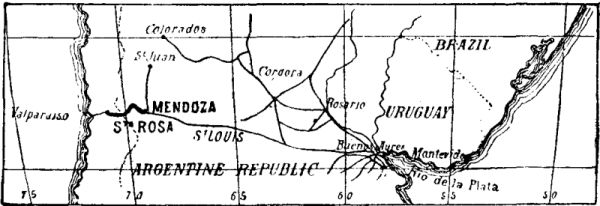
FIG. 2.—DIRECTION LINE OF THE RAILWAY THROUGH THE ANDES.
The special locomotives for use on the rack sections will weigh 45 tons in service and will haul 70 ton trains over gradients of 8 percent. Those that are to be employed upon the parts where traction will be by adhesion will be locomotives with five pairs of wheels, three of them coupled. The weight distributed over these latter will be 28 tons. These engines will haul 140 ton trains over gradients of 2 per cent.
The earthwork is now finished over two-thirds of the length, and the track has been laid for a length of 58 miles from Mendoza. It is hoped that it will be possible to open the line to traffic as far as to the summit tunnels in 1891, and to finish the tunnels in 1893. These tunnels will have to be excavated through hard rock. To this effect, it is intended to use drills actuated by electricity through dynamos driven by waterfalls. The Ferroux system seems preferable to the Brandt and other hydraulic systems, seeing the danger of the water being frozen in the conduits placed outside of the tunnels.—Le Genie Civil.
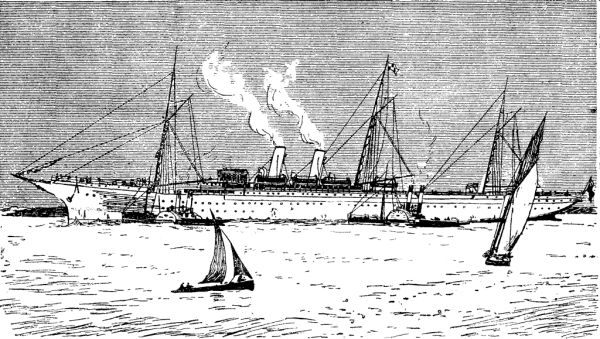
THE NEW BRITISH PACIFIC LINE EMPRESS OF INDIA.
The Empress of India is intended to be the pioneer of three fast mail steamers, built by the Barrow Shipbuilding Company for service in connection with the Canadian Pacific Railway, between Vancouver and the ports of China and Japan, thus forming the last link in the new route to the East through British territory. Her sister ships, the Empress of China and Empress of Japan, are to be ready in April next. These three ships all fulfill the requirements of the Board of Trade and of the Admiralty and Lloyd's, and are classed as 100 A1. They will also be placed on the list of British armed cruisers for service as commerce protectors in time of war. For this service each vessel is to be thoroughly fitted. There are two platforms forward and two aft, for mounting 7 in. Armstrong guns. These weapons, in the case of the Empress of India, are already awaiting the vessel at Vancouver. The Empress of India is painted white all over, has three pole masts to carry fore and aft sails. She has two buff-colored funnels and a clipper stern, and in external build much resembles the City of Rome. Her length over all is 485 feet; beam, 51 feet; depth, 36 feet; and gross tonnage, 5,920 tons. The hull, of steel, is divided into fifteen compartments by bulkheads, and has a cellular double bottom 4 feet in depth and 7 feet below the engine room. There are four complete decks. The ship is designed to carry 200 saloon passengers, 60 second cabin, and 500 steerage—these last chiefly Chinese coolies, for whose special delectation an "opium room" has been provided on board.—Daily Graphic.
The prairie land in the southwest corner of Lake Michigan, which, seventy years ago, was half morass from the overflowing of the sluggish creek, whose waters, during flood, spread over the low-lying, level plain, or were supplemented in the dry season by the inflow from the lake, showed no sign of any future development and prosperity. The few streets of wooden houses that had been built by their handful of isolated inhabitants seemed likely rather to decay from neglect and desertion than to increase, and ultimately to be swept away by fire, to make room for the extravagant and gigantic buildings that to-day characterize American civilization and commercial prosperity. Nearly 1,000 miles from the Atlantic, a greater distance from the Gulf of Mexico, and 2,000 miles from the Pacific, no wilder dream could have been imagined fifty years ago than that Chicago should become a seaport, the volume of whose business should be second only to that of New York; that forty miles of wharves and docks lining the branches of the river should be insufficient for the wants of her commerce, and that none of the magnificent lake frontage could be spared to supply the demand.
Yet this is the situation to-day, the difficulties of which must increase many fold as years pass and business grows, unless some changes are made by which increased accommodation can be obtained. The nature of these changes has long engrossed the attention of the municipality and their engineers, and necessity is forcing them from discussion to action. As such action is likely to be taken soon, the subject is of sufficient interest to the English reader to devote some space to its consideration.
The most important problem, however, which the works to be undertaken—and which must of necessity be soon commenced—will have to solve, is not one of wharf accommodation or of increased facilities of commerce. It is the better disposal of the sewage of the city, the system in use at present being inadequate, and growing more and more imperfect as the city and its population increase. During the early days of Chicago, and indeed long after, the sewage question was treated with primitive simplicity, and with a complete disregard of sanitary laws.
The river and the lake in front of the city were close at hand and convenient to receive all the discharge from the drains that flowed into them. But this condition of things had to come to an end, for the lake supplied the population with water, and it became too contaminated for use. To obtain even this temporary relief involved much of the ground level of the city being raised to a height of 14 ft. above low water, a great undertaking carried out a number of years ago. To obtain an adequate supply of pure water, Mr. E.S. Chesborough, the city engineer, adopted the ingenious plan of driving a long tunnel beneath the bed of the lake, connected at the outer end to an inlet tower built in the water, and on shore to pumping engines. This plan proved so successful that it is now being repeated on a larger scale, and with a much longer tunnel, to meet the increased demands of the large population.
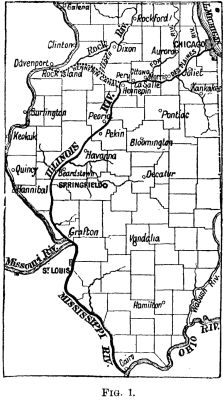
But to improve the sanitary condition of the city has been a much more difficult undertaking, as may be gathered from the following extract from an official report: "The present sanitary condition calls loudly for relief. The pollution of the Desplaines and the Illinois Rivers extends 81 miles, as far as the mouth of the Fox (see plan, Fig. 1) in summer low water, and occasionally to Peoria (158 miles) in winter. Outside of the direct circulation the river harbor is indescribable. The spewing of the harbor contents into the lake, the sewers constantly discharging therein, clouds the source of water supply (the lake) with contamination. Relief to Chicago and equity to her neighbors is a necessity of the early future." To make this quotation clear it is necessary to explain the actual condition of the Chicago sewage question.
Long before the present metropolis had arrived at the title and dignity of a city, the advantage to be derived from a waterway between Lake Michigan and the Illinois River, and thence to the Mississippi, was well understood. The scheme was, in fact, considered of sufficient importance to call for legislation as early as 1822, in which year an act was passed authorizing the construction of a canal having this object. It was not commenced, however, till 1836, and was opened to navigation in the spring of 1848. This canal extended from Chicago to La Salle, a distance of 97¼ miles, and it had a fall of 146 ft. to low water in the Illinois River (see Fig. 1). It was only a small affair, 6 ft. deep, and 60 ft. wide on the surface; the locks were 110 ft. long and 18 ft. wide. The summit level, which was only 8 ft. above the lake, was 21 miles in length. This limited waterway remained in use for a number of years, until, in fact, the growth of Chicago rendered it impossible to allow the sewage to flow any longer into the lake. In 1865 the State of Illinois sanctioned widening and lowering the canal so that it should flow by gravity from Lake Michigan. The enlargement was completed in 1871, by the city of Chicago, and the sewage was then discharged toward the Illinois River. But the flow was insufficient, and in 1881 the State called on the city to supplement the flow by pumping water into the canal.
 In 1884, engines delivering 60,000 gallons a minute
were set to work and remedied the evil for a time, so
far as the city of Chicago was concerned, but the large
discharge of sewage through the sluggish current of
the canal and into the Illinois River proved a serious
and ever-increasing nuisance to the inhabitants in the
adjoining districts. To enlarge the existing canal, increase
the volume and speed of its discharge, and to
alter the levels, so that there shall be a relatively rapid
stream flowing at all times from Lake Michigan, appears
the only practical means of affording relief to
the city, and immunity to other towns and villages lying
along the route of the stream.
In 1884, engines delivering 60,000 gallons a minute
were set to work and remedied the evil for a time, so
far as the city of Chicago was concerned, but the large
discharge of sewage through the sluggish current of
the canal and into the Illinois River proved a serious
and ever-increasing nuisance to the inhabitants in the
adjoining districts. To enlarge the existing canal, increase
the volume and speed of its discharge, and to
alter the levels, so that there shall be a relatively rapid
stream flowing at all times from Lake Michigan, appears
the only practical means of affording relief to
the city, and immunity to other towns and villages lying
along the route of the stream.
The physical nature of the country is well suited for carrying out such a project on a scale far larger than that required for sewage purposes, and works thus carried out would, to a small extent, restore the old water regime in this part of the continent. Before the vast surface changes produced during the last glacial period, three of the great lakes—Michigan, Huron and Superior—discharged their waters southward into the Gulf of Mexico by a broad river. The accumulation of glacial debris changed all this; the southern outlet was cut off, and a new one to the north was opened near where Detroit stands, making a channel to Lake Erie, which then became the outlet for the whole chain by way of Niagara. A very slight change in levels would serve to restore the present regime. Around Lake Michigan the land has been slightly raised, the summit above mean water level being only about 8 ft. Thirty miles from the south shore the lake level is again reached at a point near Lockport (see Fig. 2); the fall then becomes more marked. At Lake Joliet, 10 miles further, the fall is 77 ft.; and at La Salle, 100 miles from Chicago, the total fall reaches 146 feet. At La Salle the Illinois River is met, and this stream, after a course of 225 miles, enters the Missouri. In the whole distance the Illinois River has a fall of 29 ft. "It has a sluggish current; an oozy bed and bars, formed chiefly by tributaries, with natural depths of 2 ft. to 4 ft.; banks half way to high waters, and low bottoms, one to six miles wide, bounded by terraces, overflowed during high water from 4 ft. to 12 ft. deep, and intersected in dry seasons by lake, bayou, lagoon, and marsh, the wreck of a mighty past."
The rectification of the Illinois and the construction of a large canal from La Salle to Lake Michigan are, therefore, all that is necessary to open a waterway to the Gulf of Mexico, and to make Chicago doubly a port; on the one hand, for the enormous lake traffic now existing; on the other, for the trade that would be created in both directions, northward to Lake Michigan, and southward to the Gulf.
As a matter of fact this great scheme has long occupied the attention of the United States government. A bill in 1882 authorized surveys for "a canal from a point on the Illinois River, at or near the town of Hennepin, by the most practical route to the Mississippi River ... and a survey of the Illinois and Michigan Canal connecting the Illinois River with Chicago, and estimates from its enlargements." This scheme only contemplated navigation for boats up to 600 tons. In 1885 the Citizens' Association, of Chicago caused a report to be made for an extended plan. The name of Mr. L.E. Cooly, at that time municipal sanitary engineer, was closely associated with this report, as it is at the present time for the agitation for carrying out the works. This report recommended that "an ample channel be created from Chicago to the Illinois River, sufficient to carry away in a diluted state the sewage of a large population. That this channel may be enlarged by the State or national government to any requirement of navigation or water supply for the whole river, creating incidentally a great water power in the Desplaines valley." Following this report and that of a Drainage and Water Supply Commission, a bill was introduced into Congress supporting the recommendations that had been made, and providing the financial machinery for carrying it into execution. Since that date much discussion has taken place, and some little action; meanwhile the sanitary requirements of the city are growing more urgent, and the pressure created from this cause will enforce some decision before long. Whether the new waterway is to be practically an open sewer or a ship canal remains yet to be seen, but it is tolerably certain that its dimensions and volume of water must approximate to the latter, if the large populations of other towns are to be satisfied. In fact the actual necessities are so great as regards sectional area of canal and flow of water—at least 600,000 ft. a minute—that comparatively small extra outlay would be needed to complete the ship canal.
 The attention of engineers in Chicago, as well as of
the United States government, is consequently closely
directed at the present time to such a solution of the
problem as shall secure to Chicago such a waterway
as will dispose of the sewage question for very many
years to come; that shall relieve the inhabitants on
the line of the canal from all nuisances arising from
the sewage disposal, and shall provide a navigable
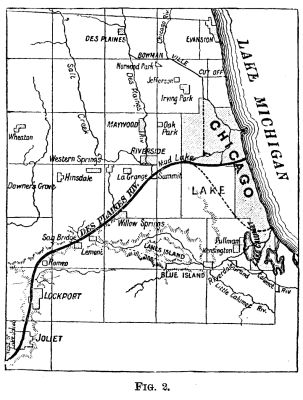
channel for vessels of deep draught. The maps, Figs.
1 and 2, give an idea of the most favored scheme—that
of Mr. Cooley.
The attention of engineers in Chicago, as well as of
the United States government, is consequently closely
directed at the present time to such a solution of the
problem as shall secure to Chicago such a waterway
as will dispose of the sewage question for very many
years to come; that shall relieve the inhabitants on
the line of the canal from all nuisances arising from
the sewage disposal, and shall provide a navigable
channel for vessels of deep draught. The maps, Figs.
1 and 2, give an idea of the most favored scheme—that
of Mr. Cooley.
As will be seen, the canal commencing near the mouth of the Chicago River passes through a cut in the low ridge forming the summit level; then it runs to Lake Joliet, and through the valleys of the Desplaines and Illinois Rivers, to the Mississippi at Grafton, a distance of 325 miles. The elevations and distances of the principal points are as follows:
| Miles from Lake Michigan. | Low Water Level below Chicago Datum. | High Water above Low Water. | ||
| ft. | ft. | |||
| Lake Michigan | 4.7 | |||
| Lake Joliet | 40 | 77 | 5 to 6 | |
| Kankakee River | 51.30 | 93.70 | 18 to 20 | |
| Morris | 61 | 100.3 | 21 | |
| Marseilles | 77 | 102.8 | 4 to 5 | |
| Ottawa | 84.5 | 132.1 | 26 | |
| La Salle | 100.3 | 146.6 | 28 | |
| Hennepin | 115.8 | 148.7 | 25 | |
| Peoria | 161.4 | 151.3 | 21 | |
| Mouth of the Illinois | 325 | 172.4 | 20 |
The project in contemplation provides that the depth of the canal as far as Lake Joliet (which is about six miles long) shall be not less than 22 ft., and on to La Salle not less than 14 ft. at first, with facilities to increase it to 22 ft. Beyond La Salle to the mouth of the Illinois, dredging and flushing by the large volume of water pouring in from Lake Michigan would make and maintain ultimately a similar depth.
As it appears recognized that the sewage channel of Chicago must be 15 ft. deep, and as provision is now being made all over the great lake system for vessels drawing 20 ft. of water, a comparatively small additional outlay would provide for a channel available for the largest lake vessels. It is claimed that by the co-operation of the Chicago municipality and the general government—the latter to advance a sum of not less than $50,000,000—a ship (and sanitary) canal 22 ft. deep could be made from the lake to Joliet, extended thence to Utica, 20 ft. deep, and from there to the Mississippi, 14 ft. deep.
That such a work would vastly enhance the commerce, not only of Chicago, but of the whole section of the country through which the canal would pass, admits of but little doubt, and probably the outlay would be justified by results similar to those achieved with other great canal works and rectified rivers in the United States.
The following figures, showing the tonnage carried in 1888-89, give some idea of the volumes of water-borne traffic in America:
| Tons. | |
| Detroit River | 19,099,060 |
| Erie Canal | 5,370,369 |
| Sault Ste. Marie | 7,516,022 |
| Welland Canal | 828,271 |
| St. Lawrence Canal | 1,500,096 |
| Mississippi to New Orleans | 3,177,000 |
| Mississippi below St. Louis | 845,000 |
| Ohio | 2,236,917 |
| Chicago Canal and lake | 11,029,575 |
Except on the Mississippi, it may be reckoned that navigation is closed by ice during five months a year. It may be mentioned, by way of comparison, that the traffic on the Suez Canal during the year 1888-89 was 6,640,834 tons.
One very interesting point in connection with this work is the effect that the diversion of so large a body of water from the lakes will have upon their regime. At least 10,000 cubic feet a second would be taken from Lake Michigan and find its way into the Mississippi; this is approximately 4½ per cent. of the total amount that now passes through the St. Clair River and thence over Niagara.
The following table gives some particulars of the great lakes and the discharge from them:
| Cubic Feet per Second | ||||||
| Lake. | Elevation above Mean Tide. | Area of Basin, Square Miles. | Area of Lake, Square Miles. | Rainfall. | Evaporation | Discharge. |
| Superior | 601.78 | 90,505 | 38,875 | 187,386 | 34,495 | 80,870 |
| Huron and Mich. | 581.28 | 121,941 | 50,400 | 262,964 | 66,754 | 216,435 |
| Erie | 572.86 | 40,298 | 10,000 | 96,654 | 13,870 | 235,578 |
| Ontario | 246.61 | 31,558 | 7,220 | 75,692 | 10,568 | 272,095 |
The average variation in level of the lakes is from 18 in. to 24 in. during the year, and the range in evaporation from year to year is also very considerable; thus the evaporation per second on Huron and Michigan, as given in the table above, is nearly 67,000 ft., but the figures for another year show nearly 89,000 ft. per second, which would represent a difference of 6½ in. in water level. As a discharge of 10,000 cubic feet a second into the new canal would lower the level of these two lakes by 2.87 in. in a year, it follows that the difference between a year of maximum and one of minimum evaporation is more than twice as great as would be required for the canal, and even under the most unfavorable conditions the volume taken from the whole chain of lakes would not lower them an inch.
When the variations in level due to different causes—rain, wind, and evaporation being the chief—are taken into consideration, the effect of 10,000 cubic feet a second abstracted would probably not be noticeable. That this would be so is the opinion, after careful investigation, of many eminent American engineers. On the other hand there is a similar unanimity of opinion as to the advantages that would be obtained in the condition of the Mississippi by adding to it a tributary of such importance as the proposed canal.—Engineering.
The inventor and patentee of all water wheels known as the Burham turbine died from Bright's disease of the kidneys at his home, York, Pa., Dec. 22, 1890, aged 68 years 9 months and 9 days. He was born in the city of New York, March 13, 1822, and was of English-Irish and French descent. His father was a millwright and with him worked at the trade in Orange county, N.Y., until he was 16 years old. He then commenced learning the watchmakers' business, which he was obliged to relinquish, after three years, on account of his health. He then went to Laurel, Md., in 1844, and engaged with Patuxent & Co. as mercantile clerk and bookkeeper. In 1856 he commenced the manufacture of the French turbine water wheel. In 1879 he sold out his Laurel interests, went to New York and commenced manufacturing his own patents. On May 22, 1883, he founded the Drovers' and Mechanics' National Bank of York, and was elected its first president, which position he held at the time of his death. In 1881, with others, he built the York opera house, at a cost of $40,000. He was a Knight Templar, and past master of the I.O.O.F., and past sachem of Red Men.
He was the oldest turbine wheel manufacturer living, having been actually engaged in the manufacture of turbines since 1856. He first made and sold the French Jonval turbine, which was then the best turbine made, but being complicated in construction, it soon wore out and leaked. From the experience he had from this wheel he invented and patented Feb. 22, 1859, his improved Jonval turbine, which was very simply constructed and yielded a greater percentage of power than the French Jonval turbines. Hundreds of these improved wheels, which were put in operation between the years 1859 and 1868, are still in use. (We show no cut of this wheel, but it had four chutes instead of six, as shown in March 24, 1863, patent.)
The first wheel (72 inch) made after the patent was granted was sold to Brightwell & Davis, Farmville, Va., and put into their flour mill under six feet head. In 1870, Brightwell & Davis sold their mill to Scott & Davis. Afterward G.W. Davis owned and operated the mill and put in one 1858 patent "New Turbine." In 1889 the Farmville Mill Company bought and remodeled the mill to roller process and required more power than the old 1856 Jonval turbine and 1868 "New Turbine" would yield, and on Aug. 30, 1889, sold the Farmville Mill Company two 54 inch new improved Standard turbines to displace the two old wheels. In 1860 he commenced experimenting with different forms of buckets and chutes, and used six chutes instead of four as first made, and was granted patent March 24, 1863.
This addition of chutes proved beneficial, as the wheel worked better with the gates partly opened than it did with four chutes. His next invention was granted him Dec. 24, 1867, which he called Burnham's improved central and vertical discharge turbine.
This improvement consisted in making the guide blade straight on the outside (instead of rounding, as then made by all others), from inner point back to bolt or gudgeon, and thick enough at the latter point to let water pass without being obstructed by said bolt and the arrangement for shifting the water guides. Two 42-inch wheels of this pattern were built and put into operation, but they soon commenced leaking water and became troublesome on account of the many small pieces of castings and bolts, and were abandoned as worthless. There are several manufacturers of this style of wheel that advertise them as "simple and durable." Such a complicated case with twelve chutes cannot be made to operate unless by a large number of castings, bolts and studs. With these adjustable water guides, one of the objects was obtained. Admitting the water to the wheel through chutes corresponding in height to the outer edge of buckets exposed, but not placing the water against the face of the buckets at right angles with the center of the wheel, except when the guide blades were full opened, for as the guides are changed so is the current of the water likewise changed.
After making several differently constructed wheels and testing them a number of times, he selected the best one and obtained a patent for it March 3, 1868, and called it "new turbine," which he still further improved and patented May 9, 1871. This "new turbine" consisted of the former improved Jonval wheel, hub and buckets, with a new circular case and new form of chutes, having a register gate entirely surrounding the case and having apertures corresponding to those in the case for admitting water to the wheel. This register gate was moved by means of a segment and pinion.
This "new turbine" soon gained for itself a reputation enjoyed by no other water wheel. It was selected by the United States Patent Office, and put at work in room 189, to run a pump which forces water to the top of the building. It was likewise selected by the Japan commission when they were in this country to select samples of our best machines. He continued making the 1868 patent and improved in 1871 "new turbine" but a few years, for as long as he could detect a defect in the wheel, case or gate, he continued improving and simplifying them, and in 1873 he erected a very complete testing flume, also made a very sensitive dynamometer, it having a combination screw for tightening the friction band, which required 100 turns to make one inch, and commenced making and experimenting with different constructed turbines. He made five different wheels and made over a hundred tests before he was satisfied. Application was then made for a patent, which was granted March 31, 1874, for his "Standard turbine."
This "Standard turbine" was a combination of his former improvements, with the cover extending over top of the gate to prevent it from tilting, and an eccentric wheel working in cam yoke to open and close the gate.
Thousands of Standard turbines are to-day working and giving the best satisfaction, and we venture to say that not one of the Standard turbines has been displaced by any other make of turbine, which gave better results for the water used. In 1881 he again commenced experimenting to find out how much water could be put through a wheel of given diameter. After making and testing several wheels it was found that the amount of water with full gate drawn named in tables found in Burnham Bros.' latest catalogue for each size wheel yielded 84 per cent. and that the water used with 7/8 gate drawn yielded the same percentage (84), or with ¾ gate 82 per cent., 5/8 gate 79, and ½ gate 75 per cent. A patent for the mechanism was applied for and granted March 27, 1883, and named Burnham's Improved Standard Turbine.
It was found that the brackets with brass rollers attached, to prevent the gate from rising and tilting and rubbing the curb, soon wore and allowed the gate to rub against the curb, and he experimented with several devices of gate arms. While so engaged he found that the great weight of water on the top of the cover sprang it, causing the sleeve bearing on the under side of the cover to be thrown out of place, and the gate pressed so hard against the case that it was almost impossible to move it, and after thoroughly testing with the different devices of gate arms, application was made and patent granted for adjustable gate arms, also for the new worm gate gearing May 1, 1888, and named Burham's new improved standard turbine.
This he improved and patented May 13, 1890, to run on horizontal shaft.
In the year 1872 he had two patents granted him for improvement in water wheels, but never had any wheels built of that pattern. After completing and patents granted for his new improved Standard turbine, he was perfectly satisfied, and often remarked, "I cannot improve on my register gate turbine any more, as it is as near perfection as can be made," and he was fully convinced, for the past year he was experimenting with a cylinder gate turbine, and patent was granted Oct. 21, 1890. Previously he had made a 24-inch wheel, which was tested Aug. 14, 1890, at Holyoke testing flume, and gave fair results, and at the time of his demise he was having made a new runner for the cylinder gate turbine, which we will complete and have tested. His idea was to have us manufacture and sell register and cylinder gate turbines. His inventive powers were not confined to water wheels, for on Feb. 23, 1886, patents were granted him for automatic steam engine, governor and lubricating device. We also remember in the year 1873 or 1874, when his mind was occupied with his "Standard turbine," he was hindered by some device used now on locomotives of the present construction (what it was we are unable to say), but when draughting at his water wheel, would conflict the two, and by his invitation we wrote to a prominent locomotive builder and had him examine the drawings, which he had not fully completed, and sold same to him. Of this we only have a faint recollection, but do recollect his saying: "Well, that is off my mind now, and I can devote it to the finishing of my new wheel."—American Miller.
At a recent meeting of the Physical Society, London, Mr. James Swinburne read a paper on alternate current condensers. It is, he said, generally assumed that there is no difficulty in making commercial condensers for high pressure alternating currents. The first difficulty is insulation, for the dielectric must be very thin, else the volume of the condenser is too great. Some dielectrics 0.2 mm. thick can be made to stand up to 8,000 volts when in small pieces, but in complete condensers a much greater margin must be allowed. Another difficulty arises from absorption, and whenever this occurs, the apparent capacity is greater than the calculated. Supposing the fibers of paper in a paper condenser to be conductors embedded in insulating hydrocarbon, then every time the condenser is charged the fibers have their ends at different potentials, so a current passes to equalize them and energy is lost. This current increases the capacity. One condenser made of paper boiled in ozokerite took an abnormally large current and heated rapidly. At a high temperature it gave off water, and the power wasted and current taken gradually decreased.
When a thin plate of mica is put between tin foils, it heats excessively; and the fall of potential over the air films separating the mica and foil is great enough to cause disruptive discharge to the surface of the mica. There appears to be a luminous layer of minute sparks under the foils, and there is a strong smell of ozone. In a dielectric which heats, there may be three kinds of conduction, viz., metallic, when an ordinary conductor is embedded in an insulator; disruptive, as probably occurs in the case of mica; and electrolytic, which might occur in glass. In a transparent dielectric the conduction must be either electrolytic or disruptive, otherwise light vibrations would be damped. The dielectric loss in a cable may be serious. Calculating from the waste in a condenser made of paper soaked in hot ozokerite, the loss in one of the Deptford mains came out 7,000 watts. Another effect observed at Deptford is a rise of pressure in the mains. There is as yet no authoritative statement as to exactly what happens, and it is generally assumed that the effect depends on the relation of capacity to self-induction, and is a sort of resonator action. This would need a large self-induction, and a small change of speed would stop the effect. The following explanation is suggested. When a condenser is put on a dynamo, the condenser current leads relatively to the electromotive force, and therefore strengthens the field magnets and increases the pressure.
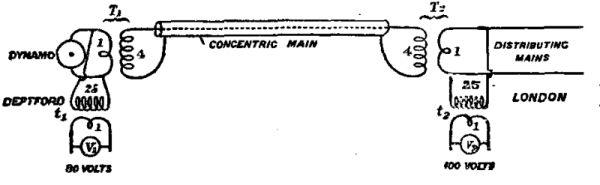
T1 and T2 are large transformers; t1 and t2 are small
transformers or voltmeters V1 and V2. The numbers
1, 4, 1, 25, represent their conversion ratios.
In order to test this, the following experiment was made for the author by Mr. W.F. Bourne. A Gramme alternator was coupled to the low pressure coil of a transformer, and a hot wire voltmeter put across the primary circuit. On putting a condenser on the high pressure circuit, the voltmeter wire fused. The possibility of making an alternator excite itself like a series machine, by putting a condenser on it, was pointed out. Prof. Perry said it would seem possible to obtain energy from an alternator without exciting the magnets independently, the field being altogether due to the armature currents. Mr. Swinburne remarked that this could be done by making the rotating magnets a star-shaped mass of iron. Sir W. Thomson thought Mr. Swinburne's estimate of the loss in the Deptford mains was rather high. He himself had calculated the power spent in charging them, and found it to be about 16 horse power, and although a considerable fraction might be lost, it would not amount to nine-sixteenths. He was surprised to hear that glass condensers heated, and inquired whether this heating was due to flashes passing between the foil and the glass. Mr. A.P. Trotter said Mr. Ferranti informed him that the capacity of his mains was about 1/3 microfarad per mile, thus making 2-1/3 microfarads for the seven miles. The heaping up of the potential only took place when transformers were used, and not when the dynamos were connected direct. In the former case the increase of volts was proportional to the length of main used, and 8,500 at Deptford gave 10,000 at London.
Mr. Blakesley described a simple method of determining the loss of power in a condenser by the use of three electrodynamometers, one of which has its coils separate. Of these coils, one is put in the condenser circuit, and the other in series with a non-inductive resistance r, shutting the condenser. If a2 be the reading of a dynamometer in the shunt circuit, and a3 that of the divided dynamometer, the power lost is given by r (Ca3 -Ba2) where B and C are the constants of the instruments on which a2 and a3 are the respective readings. Prof. S.P. Thompson asked if Mr. Swinburne had found any dielectric which had no absorption. So far as he was aware, pure quartz crystal was the only substance. Prof. Forbes said Dr. Hopkinson had found a glass which showed none. Sir William Thomson, referring to the same subject, said that many years ago he made some tests on glass bottles, which showed no appreciable absorption. Sulphuric acid was used for the coatings, and he found them to be completely discharged by an instantaneous contact of two balls. The duration of contact would, according to some remarkable mathematical work done by Hertz in 1882, be about 0.0004 second, and even this short time sufficed to discharge them completely.
On the other hand, Leyden jars with tinfoil coatings showed considerable absorption, and this he thought due to want of close contact between the foil and the glass. To test this he suggested that mercury coatings be tried. Mr. Kapp considered the loss of power in condensers due to two causes: first, that due to the charge soaking in; and second, to imperfect elasticity of the dielectric. Speaking of the extraordinary rise of pressure on the Deptford mains, he said he had observed similar effects with other cables. In his experiments the sparking distance of a 14,000 volt transformer was increased from 3/16 of an inch to 1 inch by connecting the cables to its terminals. No difference was detected between the sparking distances at the two ends of the cable, nor was any rise of pressure observed when the cables were joined direct on the dynamo.
In his opinion the rise was due to some kind of resonance, and would be a maximum for some particular frequency. Mr. Mordey mentioned a peculiar phenomenon observed in the manufacture of his alternators. Each coil, he said, was tested to double the pressure of the completed dynamo, but when they were all fitted together, their insulation broke down at the same volts. The difficulty had been overcome by making the separate coils to stand much higher pressures. Prof. Rucker called attention to the fact that dielectrics alter in volume under electric stress, and said that if the material was imperfectly elastic, some loss would result. The president said that, as some doubt existed as to what Mr. Ferranti had actually observed, he would illustrate the arrangements by a diagram. Speaking of condensers, he said he had recently tried lead plates in water to get large capacities, but so far had not been successful.
Mr. Swinburne, in replying, said he had not made a perfect condenser yet, for, although he had some which did not heat much, they made a great noise. He did not see how the rise of pressure observed by Mr. Ferranti and Mr. Kapp could be due to resonance. Mr. Kapp's experiment was not conclusive, for the length of spark is not an accurate measure of electromotive force. As regards Mr. Mordey's observation, he thought the action explicable on the theory of the leading condenser current acting on the field magnets. The same explanation is also applicable to the Deptford case, for when the dynamo is direct on, the condenser current is about 10 amperes, and this exerts only a small influence on the strongly magnetized magnets. When transformers are used, the field magnets are weak, while the condenser current rises to 40 amperes. Mr. Blakesley's method of determining losses was, he said, inapplicable except where the currents were sine functions of the time; and consequently could not be used to determine loss due to hysteresis in iron, or in a transparent dielectric.—Nature.
There are at present twenty-six submarine cable companies, the combined capital of which is about forty million pounds sterling. Their revenue, including subsidies, amounts to 3,204,060£.; and their reserves and sinking funds to 3,610,000£.; and their dividends are from one to 14¾ per cent. The receipts from the Atlantic cables alone amount to about 800,000£. annually.
The number of cables laid down throughout the world is 1,045, of which 798 belong to governments and 247 to private companies. The total length of those cables is 120,070 nautical miles, of which 107,546 are owned by private telegraph companies, nearly all British; the remainder, or 12,524 miles, are owned by governments.
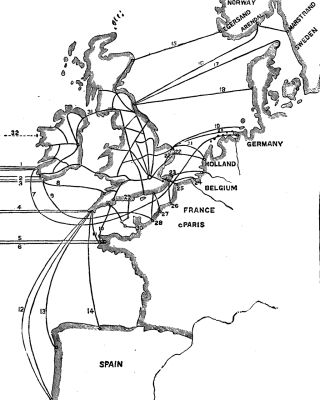 The largest telegraphic organization in the world is
that of the Eastern Telegraphic Company, with seventy
cables, of a total length of 21,859 nautical miles. The
second largest is the Eastern Extension, Australasia
and China Telegraph Company, with twenty-two
cables, of a total length of 12,958 nautical miles. The
Eastern Company work all the cables on the way to
Bombay, and the Eastern Extension Company from
Madras eastward. The cables landing in Japan, however,
are owned by a Danish company, the Great
Northern. The English station of the Eastern Company
is at Porthcurno, Cornwall, and through it pass
most of the messages for Spain, Portugal, Egypt,
India, China, Japan, and Australia.
The largest telegraphic organization in the world is
that of the Eastern Telegraphic Company, with seventy
cables, of a total length of 21,859 nautical miles. The
second largest is the Eastern Extension, Australasia
and China Telegraph Company, with twenty-two
cables, of a total length of 12,958 nautical miles. The
Eastern Company work all the cables on the way to
Bombay, and the Eastern Extension Company from
Madras eastward. The cables landing in Japan, however,
are owned by a Danish company, the Great
Northern. The English station of the Eastern Company
is at Porthcurno, Cornwall, and through it pass
most of the messages for Spain, Portugal, Egypt,
India, China, Japan, and Australia.
The third largest cable company is the Anglo-American Telegraph Company, with thirteen cables, of a total length of 10,196 miles.
The British government has one hundred and three cables around our shores, of a total length of 1,489 miles. If we include India and the colonies, the British empire owns altogether two hundred and sixteen cables of a total length of 3,811 miles.
The longest government cable in British waters is that from Sinclair Bay, Wick, to Sandwick Bay, Shetland, of the length of 122 miles, and laid in 1885. The shortest being four cables across the Gloucester and Sharpness Canal, at the latter place, and each less than 300 ft. in length.
Of government cables the greatest number is owned by Norway, with two hundred and thirty-six, averaging, however, less than a mile each in length.
The greatest mileage is owned by the government of France with 3,269 miles, of the total length of fifty-one cables.
The next being British India with 1,714 miles, and eighty-nine cables; and Germany third with 1,570 miles and forty-three cables.
Britain being fourth with ninety miles less. The oldest cable still in use is the one that was first laid, that namely from Dover to Calais. It dates from 1851.
The two next oldest cables in use being those respectively from Ramsgate to Ostend, and St. Petersburg to Cronstadt, and both laid down in 1853.
Several unsuccessful attempts were made to connect England and Ireland by means of a cable between Holyhead and Howth; but communication between the two countries was finally effected in 1853, when a cable was successfully laid between Portpatrick and Donaghadee (31).
As showing one of the dangers to which cables laid in comparatively shallow waters are exposed, we may relate the curious accident that befell the Portpatrick cable in 1873. During a severe storm in that year the Port Glasgow ship Marseilles capsized in the vicinity of Portpatrick, the anchor fell out and caught on to the telegraph cable, which, however, gave way. The ship was afterward captured and towed into Rothesay Bay, in an inverted position, by a Greenock tug, when part of the cable was found entangled about the anchor.
The smallest private companies are the Indo-European Telegraph Company, with two cables in the Crimea, of a total length of fourteen and a half miles; and the River Plate Telegraph Company, with one cable from Montevideo to Buenos Ayres, thirty-two miles long.
The smallest government telegraph organization is that of New Caledonia, with its one solitary cable one mile long.
We will now proceed to give a few particulars regarding the companies having cables from Europe to America.
The most important company is the Anglo-American Telegraph Company, whose history is inseparably connected with that of the trials and struggles of the pioneers of cable laying.
Its history begins in 1851 when Tebets, an American, and Gisborne, an English engineer, formed the Electric Telegraph Company of Newfoundland, and laid down twelve miles of cable between Cape Breton and Nova Scotia. This company was shortly afterward dissolved, and its property transferred to the Telegraphic Company of New York, Newfoundland and London, founded by Cyrus W. Field, and who in 1854 obtained an extension of the monopoly from the government to lay cables.
A cable, eighty-five miles long, was laid between Cape Breton and Newfoundland (22).
Field then came to England and floated an English company, which amalgamated with the American one under the title of the Atlantic Telegraph Company.
The story of the laying of the Atlantic cables of 1857 and 1865, their success and failures, has often been told, so we need not go into any details. It may be noted, however, that communication was first established between Valentia and Newfoundland on August 5. 1858, but the cable ceased to transmit signals on September 1, following.
During that period, ninety-seven messages had been sent from Valentia, and two hundred and sixty-nine from Newfoundland. At the present time, the ten Atlantic cables now convey about ten thousand messages daily between the two continents. The losses attending the laying of the 1865 cable resulted in the financial ruin of the Atlantic company and its amalgamation with the Anglo-American. In 1866 the Great Eastern successfully laid the first cable for the new company, and with the assistance of other vessels succeeded in picking up the broken end of the 1865 cable and completing its connection with Newfoundland.
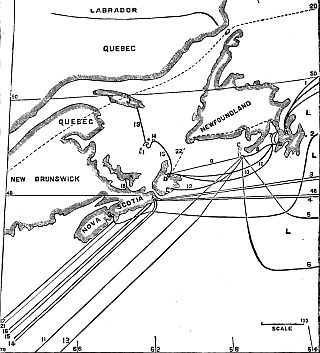
MAP SHOWING MAIN CABLES FROM EUROPE AND THEIR CONNECTIONS WITH CANADA AND THE UNITED STATES.
Reference to places—A, Heart's Content; B, Placentia; C, St. Peter Miquelon; D, North
Sydney, Cape Breton Island; E, Louisbourg; F Canso, Nova Scotia; G, Halifax; H,
Bird Rock; I, Madeline Isles; J, Anticosti; K, Charlotte Town, Prince Edward's Island;
LLL, Banks of Newfoundland.
The three cables of this company presently in use and connecting Valentia in Ireland with Heart's Content in Newfoundland, were laid in 1873, 1874, and 1880; and (1) are respectively 1886, 1846, and 1890 nautical miles in length. This company also owns the longest cable in the world, that namely from Brest in France to St. Pierre Miquelon, one of a small group of islands off the south coast of Newfoundland and which, strange to say, still belongs to France (6).
The length of this cable is 2,685 nautical miles, or 3,092 statute miles. It was laid in 1869. There are seven cables of a total length of 1773 miles, connecting Heart's Content, Placentia Bay and St. Pierre, with North Sydney, Nova Scotia, and Duxbury, near Boston, belonging to the American company. Communication is maintained with Germany and the rest of the Continent by means of a cable from Valentia to Emden 846 miles long (7); and a cable from Brest to Salcombe, Devon, connects the St. Pierre and Brest cable with the London office of the company (10).1
The station of the Direct United States Cable Company is situated at Ballinskelligs Bay, Ireland (2). Its cable was laid in 1874-5, and is 2,565 miles in length. The terminal point on the other side of the Atlantic is at Halifax, Nova Scotia, from whence the cable is continued to Rye Beach, New Hampshire, a distance of 536 miles, and thence by a land line of 500 miles to New York (17).
The Commercial Cable Company's station in Ireland is at Waterville, a short distance from Ballinskelligs (3). It owns two cables laid in 1885; the northern cable being 2,350, and the southern 2,388 miles long. They terminate in America at Canso, Nova Scotia. From Canso a cable is laid to Rockfort, about thirty miles south of Boston, Mass., a distance of 518 miles (16), and another is laid to New York, 840 miles in length (15). This company has direct communication with the Continent by means of a cable from Waterville to Havre of 510 miles (9), and with England by a cable to Weston-super-Mare, near Bristol, of 328 miles (8).
The Western Union Telegraph Company (the lessees of the lines of the American Telegraph and Cable Company) has two cables from Sennen Cove, Land's End, to Canso, Nova Scotia (4). The cable of 1881 is 2,531 and that of 1882 is 2,576 miles in length. Two cables were laid November, 1889, between Canso and New York (14).
The Compagnie Française du Telegraphe de Paris à New York has a cable from Brest to St. Pierre Miquelon of 2,242 miles in length (5), from thence a cable is laid to Louisbourg, Cape Breton (12), and another to Cape Cod (13). It has also a cable from Brest to Porcella Cove, Cornwall (11).
Those ten cables owned by the six companies named, of the total milage of 22,959, not counting connections, represent the entire direct communication between the continents of Europe and North America.
A new company, not included in the preceding statistics, proposes to lay a cable from Westport, Ireland, to some point in the Straits of Belle Isle on the Labrador coast (Map A32, Map B20).
The station of the Eastern Telegraph Company is at Porthcurno Cove, Penzance, from whence it has two cables to Lisbon, one laid in 1880, 850 miles long, the other laid in 1887, 892 miles long (12), and one cable to Vigo, Spain, laid in 1873, 622 miles long (13). From Lisbon the cable is continued to Gibraltar and the East, whither we need not follow it, our intention being to confine ourselves entirely to a brief account of those cables communicating directly with Europe and America. As already stated, this company has altogether seventy cables, of a total length of nearly 22,000 miles.
The Direct Spanish Telegraph Company has a cable, laid in 1884, from Kennach Cove, Cornwall, to Bilbao, Spain, 486 miles in length (14).
Coming now to shorter cables connecting Britain with the Continent, we have those of the Great Northern Telegraph Company, namely, Peterhead to Ekersund, Norway, 267 miles (15). Newbiggin, near Newcastle, to Arendal, Norway, 424 miles, and thence to Marstrand, Sweden, 98 miles.
Two cables from the same place in England to Denmark (Hirstals and Sondervig) of 420 and 337 miles respectively (17 and 18).
The great Northern Company has altogether twenty-two cables, of a total length of 6,110 miles. The line from Newcastle, is worked direct to Nylstud, in Russia—a distance of 890 miles—by means of a "relay" or "repeater," at Gothenburg. The relay is the apparatus at which the Newcastle current terminates, but in ending there it itself starts a fresh current on to Russia.
The other continental connections belong to the government, and are as follows: two cables to Germany, Lowestoft to Norderney, 232 miles, and to Emden, 226 miles (19 and 20).
Two cables to Holland: Lowestoft to Zandvoort, laid in 1858 (21), and from Benacre, Kessingland, to Zandvoort (22).
Two cables to Belgium: Ramsgate to Ostend (23), and Dover to Furness (24).
Four cables to France: Dover to Calais, laid in 1851 (25), and to Boulogne (26), laid in 1859; Beachy Head to Dieppe (27), and to Havre (28).
There is a cable from the Dorset coast to Alderney and Guernsey, and from the Devon coast to Guernsey, Jersey, and Coutances, France (29 and 30).
A word now as to the instruments used for the transmission of messages. Those for cables are of two kinds, the mirror galvanometer and the siphon recorder, both the product of Sir Wm. Thomson's great inventive genius.
When the Calais-Dover and other short cables were first worked, it was found that the ordinary needle instrument in use on land lines was not sufficiently sensitive to be affected trustworthily by the ordinary current it was possible to send through a cable. Either the current must be increased in strength or the instruments used must be more sensitive. The latter alternative was chosen, and the mirror galvanometer was the result.
The principle on which this instrument works may be briefly described thus: the transmitted current of electricity causes the deflection of a small magnet, to which is attached a mirror about three-eighths of an inch in diameter, a beam of light is reflected from a properly arranged lamp, by the mirror, on to a paper scale. The dots and dashes of the Morse code are indicated by the motions of the spot of light to the right and left respectively of the center of the scale.
The mirror galvanometer is now almost entirely superseded by the siphon recorder. This is a somewhat complicated apparatus, with the details of which we need not trouble our readers. Suffice it for us to explain that a suspended coil is made to communicate its motions, by means of fine silk fibers, to a very fine glass siphon, one end of which dips into an insulated metallic vessel containing ink, while the other extremity rests, when no current is passing, just over the center of a paper ribbon. When the instrument is in use the ink is driven out of the siphon in small drops by means of an electrical arrangement, and the ribbon underneath is at the same time caused to pass underneath its point by means of clockwork.
If a current be now sent through the line, the siphon will move above or below the central line, thus giving a permanent record of the message, which the mirror instrument does not. The waves written by the siphon above the central line corresponding to the dots of the Morse code, and the waves underneath corresponding to the dashes.
The cost of the transmission of a cablegram varies from one shilling per word, the rate to New York and east of the Mississippi, to ten shillings and seven pence per word, the rate to New Zealand. In order to minimize that cost as much as possible, the use of codes, whereby one word is made to do duty for a lengthy phrase, is much resorted to. Of course those code messages form a series of words having no apparent relation to each other, but occasionally queer sentences result from the chance grouping of the code words. Thus a certain tea firm was once astonished to receive from its agent abroad the startling code message—"Unboiled babies detested"!
Suppose we now follow the adventures of a few cablegrams in their travels over the world.
A message to India from London by the cable route requires to be transmitted eight times at the following places: Porthcurno (Cornwall), Lisbon, Gibraltar, Malta, Alexandria, Suez, Aden, Bombay.
A message to Australia has thirteen stoppages; the route taken beyond Bombay being via Madras, Penang, Singapore, Banjoewangie and Port Darwin (North Australia); or from Banjoewangie to Roebuck Bay (Western Australia).
To India by the Indo-European land lines, messages go through Emden, Warsaw, Odessa, Kertch, Tiflis, Teheran, Bushire (Persian Gulf), Jask and Kurrachee, but only stop twice between London and Teheran—namely, at Emden and Odessa. Messages from London to New York are transmitted only twice—at the Irish or Cornwall stations, and at the stations in Canada. Owing to the great competition for the American traffic, the service between London, Liverpool, and Glasgow and New York is said to be much superior to that between any two towns in Britain. The cables are extensively used by stock brokers, and it is a common occurrence for one to send a message and receive a reply within five minutes.
During breakages in cables messages have sometimes to take very circuitous routes. For instance, during the two days, three years ago, that a tremendous storm committed such havoc among the telegraph wires around London, cutting off all communication with the lines connected with the Channel cables at Dover, Lowestoft, etc., it was of common occurrence for London merchants to communicate with Paris through New York. The cablegram leaving London going north to Holyhead and Ireland, across the Atlantic to New York and back via St. Pierre to Brest and thence on to Paris, a total distance of about seven thousand miles.
Three years ago, when the great blizzard cut off all communication between New York and Boston, messages were accepted in New York, sent to this country, and thence back to Boston.
Some time ago the cables between Madeira and St. Vincent were out of order, cutting off communication by the direct route to Brazil, and a message to reach Rio Janeiro had to pass through Ireland, Canada, United States, to Galveston, thence to Vera Cruz, Guatemala, Nicaragua, Panama, Ecuador, Peru, Chili; from Valparaiso across the Andes, through the Argentine Republic to Buenos Ayres, and thence by East Coast cables to Rio Janeiro, the message having traversed a distance of about twelve thousand miles and having passed through twenty-four cables and some very long land lines, instead of passing, had it been possible to have sent it by the direct route, over one short land line and six cables, in all under six thousand miles.
Perhaps some of our readers may remember having read in the newspapers of the result of last year's Derby having been sent from Epsom to New York in fifteen seconds, and may be interested to know how it was done. A wire was laid from near the winning post on the race course to the cable company's office in London, and an operator was at the instrument ready to signal the two or three letters previously arranged upon for each horse immediately the winner had passed the post. When the race began, the cable company suspended work on all the lines from London to New York and kept operators at the Irish and Nova Scotian stations ready to transmit the letters representing the winning horse immediately, and without having the message written out in the usual way. When the race was finished, the operator at Epsom at once sent the letters representing the winner, and before he had finished the third letter, the operator in London had started the first one to Ireland. The clerk in Ireland immediately on bearing the first signal from London passed it on to Nova Scotia, from whence it was again passed on to New York. The result being that the name of the winner was actually known in New York before the horses had pulled up after passing the judge. It seems almost incredible that such information could be transmitted such a great distance in fifteen seconds, but when we get behind the scenes and see exactly how it is accomplished, and see how the labor and time of signaling can be economized, we can easily realize the fact.
The humors of telegraphic mistakes have often been described; we will conclude by giving only one example. A St. Louis merchant had gone to New York on business, and while there received a telegram from the family doctor, which ran: "Your wife has had a child, if we can keep her from having another to-night, all will be well." As the little stranger had not been expected, further inquiry was made and elicited the fact that his wife had simply had a "chill"! This important difference having been caused simply by the omission of a single dot.
-.-. .... .. .-.. .-..
c h i l l = chill
-.-. .... .. .-.. -..
c h i l d = child
—Hardwicke's Science-Gossip.
Cables not fully described in the text, Map B. Eight cables at the Anglo-American Company: 7, Heart's Content to Placentia, two cables; 8, Placentia to St. Pierre; 9, St. Pierre to North Sydney; 10, Placentia to North Sydney, two cables; 11, St. Pierre to Duxbury; 18, Charlotte's Town to Nova Scotia; 19, Government Cable, North Sydney to Bird Rock, Madeline Isles, and Anticosti; 21, Halifax and Bermuda Cable Company's proposed cable to Bermuda.
If an idle pole, C, C, Fig. 12 (P=0.0001 millimeter or 0.13 M), protected all but the point by a thick coating of glass, is brought into the center of the molecular stream in front of the negative pole, A, and the whole of the inside and outside of the tube walls are coated with metal, D, D, and "earthed" so as to carry away the positive electricity as rapidly as possible, then it is seen that the molecules leaving the negative pole and striking upon the idle pole, C, on their journey along the tube carry a negative charge and communicate negative electricity to the idle pole.
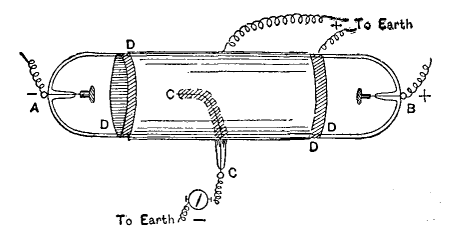
FIG. 12.—PRESSURE = 0.0001 MM. = 0.13 M.
This tube is of interest, since it is the one in which I was first able to perceive how, in my earlier results, I always obtained a positive charge from an idle pole placed in the direct stream from the negative pole. Having got so far, it was easy to devise a form of apparatus that completely verified the theory, and at the same time threw considerably more light upon the subject. Fig. 13, a, b, c, is such a tube, and in this model I have endeavored to show the electrical state of it at a high vacuum by marking a number of + and - signs. The exhaustion has been carried to 0.0001 millimeter, or 0.13 M, and you see that in the neighborhood of the positive pole, and extending almost to the negative, the tube is strongly electrified with positive electricity, the negative atoms shooting out from the negative pole in a rapidly diminishing cone. If an idle pole is placed in the position shown at Fig. 13, a, the impacts of positive and negative molecules are about equal, and no decided current will pass from it, through the galvanometer, to earth. This is the neutral point. But if we imagine the idle pole to be as at Fig. 13, b, then the positively electrified molecules greatly preponderate over the negative molecules, and positive electricity is shown. If the idle pole is now shifted, as shown at Fig. 13, c, the negative molecules preponderate, and the pole will give negative electricity.
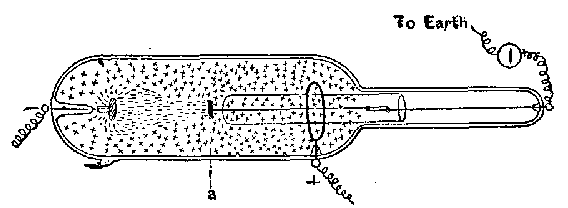
FIG. 13 A.—PRESSURE = 0.0001 MM. = 0.13 M
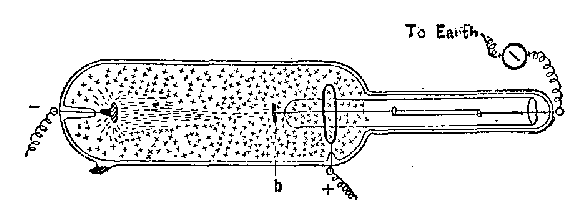
FIG. 13 B.—PRESSURE = 0.0001 MM. = 0.13 M.
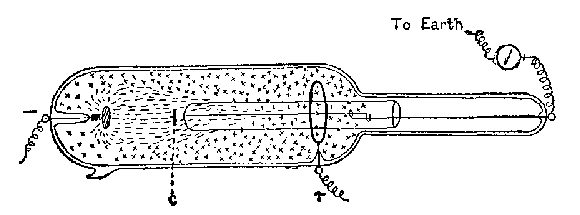
FIG. 13 C.—PRESSURE = 0.0001 MM. = 0.13 M.
As the exhaustion proceeds, the positive charge in the tube increases and the neutral point approaches closer to the negative pole, and at a point just short of non-conduction so greatly does the positive electrification preponderate that it is almost impossible to get negative electricity from the idle pole, unless it actually touches the negative pole. This tube is before you, and I will now proceed to show the change in direction of current by moving the idle pole.
I have not succeeded in getting the "Edison" current incandescent lamps to change in direction at even the highest degree of exhaustion which my pump will produce. The subject requires further investigation, and like other residual phenomena these discrepancies promise a rich harvest of future discoveries to the experimental philosopher, just as the waste products of the chemist have often proved the source of new and valuable bodies.
One of the most characteristic attributes of radiant matter—whence its name—is that it moves in approximately straight lines and in a direction almost normal to the surface of the electrode. If we keep the induction current passing continuously through a vacuum tube in the same direction, we can imagine two ways in which the action proceeds: either the supply of gaseous molecules at the surface of the negative pole must run short and the phenomena come to an end, or the molecules must find some means of getting back. I will show you an experiment which reveals the molecules in the very act of returning. Here is a tube (Fig. 14) exhausted to a pressure of 0.001 millimeter or 1.3 M. In the middle of the tube is a thin glass diaphragm, C, pierced with two holes, D and E. At one part of the tube a concave pole, A', is focused on the upper hole, D, in the diaphragm. Behind the upper hole and in front of the lower one are movable vanes, F and G, capable of rotation by the slightest current of gas through the holes.
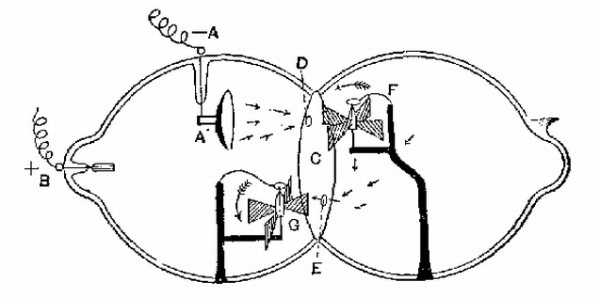
FIG. 14—PRESSURE = 0.001 MM. = 1.3 M.
On passing the current with the concave pole negative, the small veins rotate in such a manner as to prove that at this high exhaustion a stream of molecules issues from the lower hole in the diaphragm, while at the same time a stream of freshly charged molecules is forced by the negative pole through the upper hole. The experiment speaks for itself, showing as forcibly as an experiment can show that so far the theory is right.
This view of the ultra-gaseous state of matter is advanced merely as a working hypothesis, which, in the present state of our knowledge, may be regarded as a necessary help to be retained only so long as it proves useful. In experimental research early hypotheses have necessarily to be modified, or adjusted, or perhaps entirely abandoned, in deference to more accurate observations. Dumas said, truly, that hypotheses were like crutches, which we throw away when we are able to walk without them.
In recording my investigations on the subject of radiant matter and the state of gaseous residues in high vacua under electrical strain, I must refer to certain attacks on the views I have propounded. The most important of these questionings are contained in a volume of "Physical Memoirs," selected and translated from foreign sources under the direction of the Physical Society (vol. i., part 2). This volume contains two memoirs, one by Hittorff on the "Conduction of Electricity in Gases," and the other by Puluj on "Radiant Electrode Matter and the So-called Fourth State." Dr. Puluj's paper concerns me most, as the author has set himself vigorously to the task of opposing my conclusions. Apart from my desire to keep controversial matter out of an address of this sort, time would not permit me to discuss the points raised by my critic; I will, therefore, only observe in passing that Dr. Puluj has no authority for linking my theory of a fourth state of matter with the highly transcendental doctrine of four dimensional space.
Reference has already been made to the mistaken supposition that I have pronounced the thickness of the dark space in a highly exhausted tube through which an induction spark is passed to be identical with the natural mean free path of the molecules of gas at that exhaustion. I could quote numerous passages from my writings to show that what I meant and said was the mean free path as amplified and modified by the electrification.2 In this view I am supported by Prof. Schuster,3 who, in a passage quoted below, distinctly admits that the mean free path of an electrified molecule may differ from that of one in its ordinary state.
The great difference between Puluj and me lies in his statement that4 "the matter which fills the dark space consists of mechanical detached particles of the electrodes which are charged with statically negative electricity, and move progressively in a straight direction."
To these mechanically detached particles of the electrodes, "of different sizes, often large lumps,"5 Puluj attributes all the phenomena of heat, force and phosphorescence that I from time to time have described in my several papers.
Puluj objects energetically to my definition "Radiant Matter," and then proposes in its stead the misleading term "Radiant Electrode Matter." I say "misleading," for while both his and my definitions equally admit the existence of "Radiant Matter," he drags in the hypothesis that the radiant matter is actually the disintegrated material of the poles.
Puluj declares that the phenomena I have described in high vacua are produced by his irregularly shaped lumps of radiant electrode matter. My contention is that they are produced by radiant matter of the residual molecules of gas.
Were it not that in this case we can turn to experimental evidence, I would not mention the subject to you. On such an occasion as this controversial matter must have no place; therefore I content myself at present by showing a few novel experiments which demonstratively prove my case.
Let me first deal with the radiant electrode hypothesis. Some metals, it is well known, such as silver, gold or platinum, when used for the negative electrode in a vacuum tube, volatilize more or less rapidly, coating any object in their neighborhood with a very even film. On this depends the well known method of electrically preparing small mirrors, etc. Aluminum, however, seems exempt from this volatility. Hence, and for other reasons, it is generally used for electrodes.
If, then, the phenomena in a high vacuum are due to the "electrode matter," the more volatile the metal used, the greater should be the effect.6
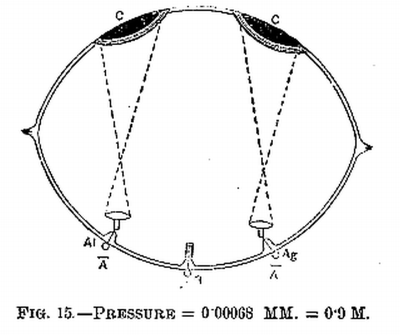 Here is a tube (Fig. 15, P=0.00068 millimeter, or
0.9 M), with two negative electrodes, AA', so placed as
to protect two luminous spots on the phosphorescent
glass of the tube. One electrode, A', is of pure silver,
a volatile metal; the other, A, is of aluminum, practically
non-volatile. A quantity of "electrode matter"
will be shot off from the silver pole, and practically
none from the aluminum pole; but you see that in
each case the phosphorescence, CC', is identical. Had
the radiant electrode matter been the active agent, the
more intense phosphorescence would proceed from the
more volatile pole.
Here is a tube (Fig. 15, P=0.00068 millimeter, or
0.9 M), with two negative electrodes, AA', so placed as
to protect two luminous spots on the phosphorescent
glass of the tube. One electrode, A', is of pure silver,
a volatile metal; the other, A, is of aluminum, practically
non-volatile. A quantity of "electrode matter"
will be shot off from the silver pole, and practically
none from the aluminum pole; but you see that in
each case the phosphorescence, CC', is identical. Had
the radiant electrode matter been the active agent, the
more intense phosphorescence would proceed from the
more volatile pole.
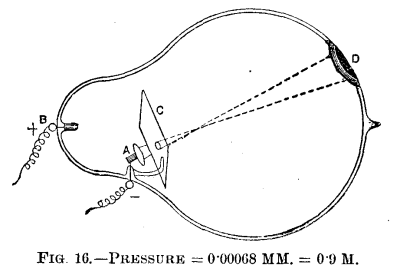 A drawing of another experimental piece of apparatus
is shown in Fig. 16. A pear-shaped bulb of German
glass has near the small end an inner concave negative
pole, A, of pure silver, so mounted that its inverted
image is thrown upon the opposite end of the tube.
In front of this pole is a screen of mica, C, having a
small hole in the center, so that only a narrow pencil
of rays from the silver pole can pass through, forming
a bright spot, D, at the far end of the bulb. The exhaustion
is about the same as in the previous tube, and
the current has been allowed to pass continuously for
many hours so as to drive off a certain portion of the
silver electrode; and upon examination it is found that
the silver has all been deposited in the immediate
neighborhood of the pole; while the spot, D, at the far
end of the tube, that has been continuously glowing
with phosphorescent light, is practically free from
silver.
A drawing of another experimental piece of apparatus
is shown in Fig. 16. A pear-shaped bulb of German
glass has near the small end an inner concave negative
pole, A, of pure silver, so mounted that its inverted
image is thrown upon the opposite end of the tube.
In front of this pole is a screen of mica, C, having a
small hole in the center, so that only a narrow pencil
of rays from the silver pole can pass through, forming
a bright spot, D, at the far end of the bulb. The exhaustion
is about the same as in the previous tube, and
the current has been allowed to pass continuously for
many hours so as to drive off a certain portion of the
silver electrode; and upon examination it is found that
the silver has all been deposited in the immediate
neighborhood of the pole; while the spot, D, at the far
end of the tube, that has been continuously glowing
with phosphorescent light, is practically free from
silver.
The experiment is too lengthy for me to repeat it here, so I shall not attempt it; but I have on the table the results for examination.
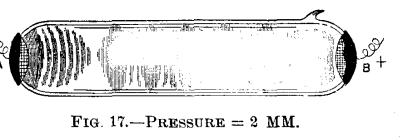 The identity of action of silver and aluminum in the
first case, and the non-projection of silver in this second
instance, are in themselves sufficient to condemn
Dr. Puluj's hypotheses, since they prove that phosphorescence
is independent of the material of the negative
electrode. In front of me is a set of tubes that
to my mind puts the matter wholly beyond doubt.
The tubes contain no inside electrodes with the residual
gaseous molecules; and with them I will proceed
to give some of the most striking radiant-matter experiments
without any inner metallic poles at all.
The identity of action of silver and aluminum in the
first case, and the non-projection of silver in this second
instance, are in themselves sufficient to condemn
Dr. Puluj's hypotheses, since they prove that phosphorescence
is independent of the material of the negative
electrode. In front of me is a set of tubes that
to my mind puts the matter wholly beyond doubt.
The tubes contain no inside electrodes with the residual
gaseous molecules; and with them I will proceed
to give some of the most striking radiant-matter experiments
without any inner metallic poles at all.
In all these tubes the electrodes, which are of silver,
are on the outside, the current acting through the body
of the glass. The first tube contains gas only slightly
rarefied and at the stratification stage. It is simply a
closed glass cylinder, with a coat of silver deposited
outside at each end, and exhausted to a pressure of 2
millimeters. The outline of the tube is shown in Fig.
17. I pass a current, and, as you see, the stratifications,
though faint, are perfectly formed.
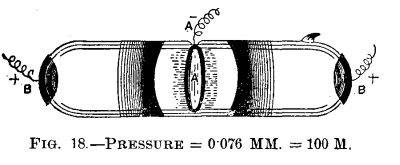
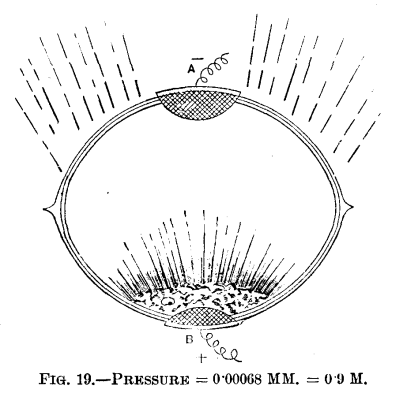 The next tube, seen in outline in Fig. 18, shows the
dark space. Like the first it is a closed cylinder of
glass, with a central indentation forming a kind of
hanging pocket and almost dividing the tube into two
compartments. This pocket, silvered on the air side,
forms a hollow glass diaphragm that can be connected
electrically from the outside, forming the negative
pole, A; the two ends of the tube, also outwardly
silvered, form the positive poles, B B. I pass the current,
and you will see the dark space distinctly visible.
The pressure here is 0.076 millimeter, or 100 M. The
next stage, dealing with more rarefied matter, is that
of phosphorescence. Here is an egg-shaped bulb,
shown in Fig 19, containing some pure yttria and a
few rough rubies. The positive electrode, B, is on the
bottom of the tube under the phosphorescent material;
the negative, A, is on the upper part of the tube. See
how well the rubies and yttria phosphorescence shows
under molecular bombardment, at an internal pressure
of 0.00068 millimeter, or 0.9 M.
The next tube, seen in outline in Fig. 18, shows the
dark space. Like the first it is a closed cylinder of
glass, with a central indentation forming a kind of
hanging pocket and almost dividing the tube into two
compartments. This pocket, silvered on the air side,
forms a hollow glass diaphragm that can be connected
electrically from the outside, forming the negative
pole, A; the two ends of the tube, also outwardly
silvered, form the positive poles, B B. I pass the current,
and you will see the dark space distinctly visible.
The pressure here is 0.076 millimeter, or 100 M. The
next stage, dealing with more rarefied matter, is that
of phosphorescence. Here is an egg-shaped bulb,
shown in Fig 19, containing some pure yttria and a
few rough rubies. The positive electrode, B, is on the
bottom of the tube under the phosphorescent material;
the negative, A, is on the upper part of the tube. See
how well the rubies and yttria phosphorescence shows
under molecular bombardment, at an internal pressure
of 0.00068 millimeter, or 0.9 M.
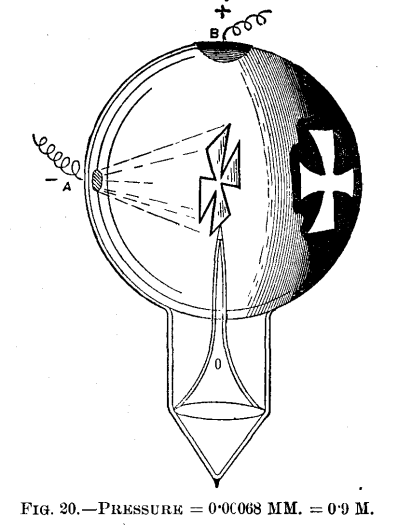 A shadow of an object inside a bulb can also be projected
on to the opposite wall of the bulb by means of
an outside pole. A mica cross is supported in the middle
of the bulb (Fig. 20), and on connecting a small
silvered patch, A, on one side of the bulb with the
negative pole of the induction coil, and putting the
positive pole to another patch of silver, B, at the top,
the opposite side of the bulb glows with a phosphorescent
light, on which the black shadow of the cross
seems sharply cut out. Here the internal pressure is
0.00068 millimeter, or 0.9 M.
A shadow of an object inside a bulb can also be projected
on to the opposite wall of the bulb by means of
an outside pole. A mica cross is supported in the middle
of the bulb (Fig. 20), and on connecting a small
silvered patch, A, on one side of the bulb with the
negative pole of the induction coil, and putting the
positive pole to another patch of silver, B, at the top,
the opposite side of the bulb glows with a phosphorescent
light, on which the black shadow of the cross
seems sharply cut out. Here the internal pressure is
0.00068 millimeter, or 0.9 M.
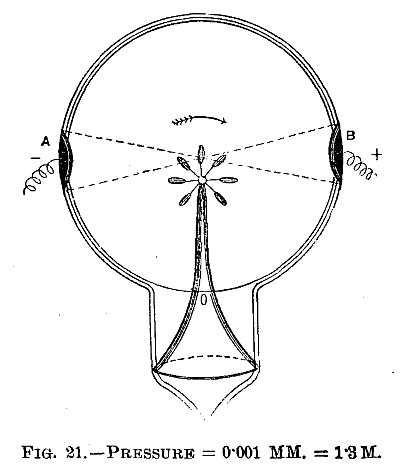 Passing to the next phenomenon, I proceed to show
the production of mechanical energy in a tube without
internal poles. It is shown in Fig. 21 (P = 0.001
millimeter, or 1.3 M). It contains a light wheel of
aluminum, carrying vanes of transparent mica, the
poles, A B, being in such a position outside that the
molecular focus falls upon the vanes on one side only.
The bulb is placed in the lantern and the image is projected
on the screen; if I now pass the current, you
see the wheels rotate rapidly, reversing in direction as
I reverse the current.
Passing to the next phenomenon, I proceed to show
the production of mechanical energy in a tube without
internal poles. It is shown in Fig. 21 (P = 0.001
millimeter, or 1.3 M). It contains a light wheel of
aluminum, carrying vanes of transparent mica, the
poles, A B, being in such a position outside that the
molecular focus falls upon the vanes on one side only.
The bulb is placed in the lantern and the image is projected
on the screen; if I now pass the current, you
see the wheels rotate rapidly, reversing in direction as
I reverse the current.
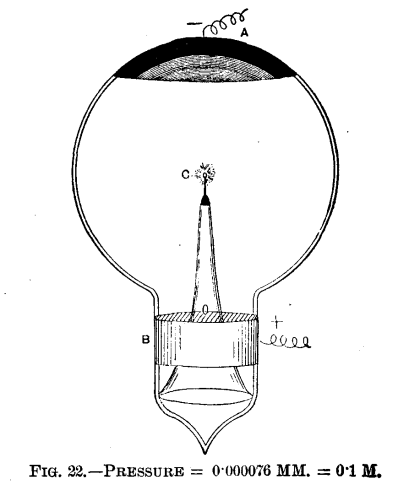 Here is an apparatus (Fig. 22) which shows that the
residual gaseous molecules when brought to a focus
produce heat. It consists of a glass tube with a bulb
blown at one end and a small bundle of carbon wool,
C, fixed in the center, and exhausted to a pressure of
0.000076 millimeter, or 0.1 M. The negative electrode,
A, is formed by coating part of the outside of the bulb
with silver, and it is in such a position that the focus
of rays falls upon the carbon wool. The positive electrode,
B, is an outer coating at the other end of the
tube. I pass the current, and those who are close may
see the bright sparks of carbon raised to incandescence
by the impact of the molecular stream.
Here is an apparatus (Fig. 22) which shows that the
residual gaseous molecules when brought to a focus
produce heat. It consists of a glass tube with a bulb
blown at one end and a small bundle of carbon wool,
C, fixed in the center, and exhausted to a pressure of
0.000076 millimeter, or 0.1 M. The negative electrode,
A, is formed by coating part of the outside of the bulb
with silver, and it is in such a position that the focus
of rays falls upon the carbon wool. The positive electrode,
B, is an outer coating at the other end of the
tube. I pass the current, and those who are close may
see the bright sparks of carbon raised to incandescence
by the impact of the molecular stream.
You thus have seen that all the old "radiant matter"
effects can be produced in tubes containing no metallic
electrodes to volatilize. It may be suggested that the
sides of the tube in contact with the outside poles become
electrodes in this case, and that particles of the
glass itself may be torn off and projected across, and
so produce the effects. This is a strong argument,
which fortunately can be tested by experiment. In
the case of this tube (Fig. 23, P = 0.00068 millimeter,
or 0.9 M), the bulb is made of lead glass phosphorescing
blue under molecular bombardment. Inside
the bulb, completely covering the part that would
form the negative pole, A, I have placed a substantial
coat of yttria, so as to interpose a layer of this earth
between the glass and the inside of the tube. The
negative and positive poles are silver disks on the outside
of the bulb, A being the negative and B the positive
poles. If, therefore, particles are torn off and
projected across the tube to cause phosphorescence,
these particles will not be particles of glass, but of
yttria; and the spot of phosphorescent light, C, on
the opposite side of the bulb will not be the dull blue
of lead glass, but the golden yellow of yttria. You see
there is no such indication; the glass phosphoresces
with its usual blue glow, and there is no evidence that
a single particle of yttria is striking it.
Witnessing these effects I think you will agree I am justified in adhering to my original theory, that the phenomena are caused by the radiant matter of the residual gaseous molecules, and certainly not by the torn-off particles of the negative electrode.
I have already pointed out that the molecular motions rendered visible in a vacuum tube are not the motions of molecules under ordinary conditions, but are compounded of these ordinary or kinetic motions and the extra motion due to the electrical impetus.
Experiments show that in such tubes a few molecules may traverse more than a hundred times the mean free path, with a correspondingly increased velocity, until they are arrested by collisions. Indeed, the molecular free path may vary in one and the same tube, and at one and the same degree of exhaustion.
Very many bodies, such as ruby, diamond, emerald, alumina, yttria, samaria, and a large class of earthy oxides and sulphides, phosphoresce in vacuum tubes when placed in the path of the stream of electrified molecules proceeding from the negative pole. The composition of the gaseous residue present does not affect phosphorescence; thus, the earth yttria phosphoresces well in the residual vacua of atmospherical air, of oxygen, nitrogen, carbonic anhydride, hydrogen, iodine, sulphur and mercury.
With yttria in a vacuum tube, the point of maximum phosphorescence, as I have already pointed out, lies on the margin of the dark space. The diagram (Fig. 24) shows approximately the degree of phosphorescence in different parts of a tube at an internal pressure of 0.25 millimeter, or 330 M. On the top you see the positive and negative poles, A and B, the latter having the outline of the dark space shown by a dotted line, C. The curve, D E F, shows the relative intensities of the phosphorescence at different distances from the negative pole, and the position inside the dark space at which phosphorescence does not occur. The height of the curve represents the degree of phosphorescence. The most decisive effects of phosphorescence are reached by making the tube so large that the walls are outside the dark space, while the material submitted to experiment is placed just at the edge of the dark space.
FIG. 24—PRESSURE = 0.25 MM. = 330 M.
Hitherto I have spoken only of the phosphorescence of substances placed under the negative pole. But from numerous experiments I find that bodies will phosphoresce in actual contact with the negative pole.
This is only a temporary phenomenon, and ceases entirely when the exhaustion is pushed to a very high point. The experiment is one scarcely possible to exhibit to an audience, so I must content myself with describing it. A U-tube, shown in Fig. 25, has a flat aluminum pole, in the form of a disk, at each end, both coated with a paint of phosphorescent yttria. As the rarefaction approaches about 0.5 millimeter the surface of the negative pole, A, becomes faintly phosphorescent. On continuing the exhaustion this luminosity rapidly diminishes, not only in intensity but in extent, contracting more and more from the edge of the disk, until ultimately it is visible only as a bright spot in the center. This fact does not prop a recent theory, that as the exhaustion gets higher the discharge leaves the center of the pole and takes place only between the edge and the walls of the tube.
FIG. 25
If the exhaustion is further pushed, then, at the point where the surface of the negative pole ceases to be luminous, the material on the positive pole, B, commences to phosphoresce, increasing in intensity until the tube refuses to conduct, its greatest brilliancy being just short of this degree of exhaustion. The probable explanation is that the vagrant molecules I introduce in the next experiment, happening to come within the sphere of influence of the positive pole, rush violently to it, and excite phosphorescence in the yttria, while losing their negative charge.
Presidential address before the Institute of Electrical Engineers, London; continued from SUPPLEMENT, No. 792, page 12656.
"The thickness of the dark space surrounding the negative pole is the measure of the mean length of the path of the gaseous molecules between successive collisions. The electrified molecules are projected from the negative pole with enormous velocity, varying, however, with the degree of exhaustion and intensity of the induction current."—Phil. Trans., part i., 1879, par. 530.
"The extra velocity with which the molecules rebound from the excited negative pole keeps back the more slowly moving molecules which are advancing toward the pole. The conflict occurs at the boundary of the dark space, where the luminous margin bears witness to the energy of the discharge."—Phil. Trans., part i., 1879, par. 507.
"Here, then, we see the induction spark actually illuminating the lines of molecular pressure caused by the excitement of the negative pole."—R.I. Lecture, Friday, April 4, 1879.
"The electrically excited negative pole supplies the force majeure, which entirely, or partially, changes into a rectilinear action the irregular vibration in all directions."—Proc. Roy. Soc., 1880. page 472.
"It is also probable that the absolute velocity of the molecules is increased so as to make the mean velocity with which they leave the negative pole greater than that of ordinary gaseous molecules."—Phil. Trans., part ii., 1881, par. 719.]
"It has been suggested that the extent of the dark space represents the mean free path of the molecules.... It has been pointed out by others that the extent of the dark space is really considerably greater than the mean free path of the molecules, calculated according to the ordinary way. My measurements make it nearly twenty times as great. This, however, is not in itself a fatal objection; for, as we have seen, the mean free path of an ion may be different from that of a molecule moving among others."—Schuster, Proc. Roy. Soc., xlvii., pp. 556-7.
"Physical Memoirs," part ii., vol. i., p. 244. The paragraph is italicized in the original.
In a valuable paper read before the Royal Society, November 20, 1890, by Professors Liveing and Dewar, on finely divided metallic dust thrown off the surface of various electrodes, in vacuum tubes, they find not only that dust, however fine, suspended in a gas will not act like gaseous matter in becoming luminous with its characteristic spectrum in an electric discharge, but that it is driven with extraordinary rapidity out of the course of the discharge.
[Continued from SUPPLEMENT, No. 794, page 12690.]
Having now brought before you the various methods by which ordinary coal gas can be enriched, so as to give an increased luminosity to the flame, I wish now to discuss the methods by which the gas can be burnt, in order to yield the greatest amount of light, and also the compounds which are produced during combustion.
In the first lecture, while discussing the theory of luminous flames, I pointed out that, in an atmospheric burner, it was not the oxygen of the air introduced combining with and burning up the hydrocarbons, and so preventing the separation of incandescent carbon, which gave the non-luminous flame, but the diluting action of the nitrogen, which acted by increasing the temperature at which the hydrocarbons are broken up, and carbon liberated, a fact which was proved by observation that heating the mixture of gas and air again restored the luminosity of the flame. This experiment clearly shows that temperature is a most important factor in the illuminating value of a flame, and this is still further shown by a study of the action of the diluents present in coal gas, the non-combustible ones being far more deleterious than the combustible, as they not only dilute, but withdraw heat.
Anything which will increase the temperature of the flame will also increase the illuminating power, provided, of course, that the increase in temperature is not, obtained at the expense of the too rapid combustion of the hydrocarbons.
As has been shown in the experiments relating to the action of diluents on flame, already quoted, oxygen, when added to coal gas, increases its illuminating value to a marked and increasing degree, until a certain percentage has been added, after which the illuminating power is rapidly decreased, until the point is reached when the mixture becomes explosive. This is due to the fact that the added oxygen increases the temperature of the flame by doing the work of the air, but without the cooling and diluting action of the nitrogen; when, however, a certain proportion is added, it begins to burn up the heavy hydrocarbons, and although the temperature goes on increasing, the light-giving power is rapidly diminished by the diminution of the amount of free carbon in the flame.
It has been proposed to carburet and enrich poor coal gas by admixture with it of an oxy-oil gas made under Tatham's patents, in which crude oils are cracked at a comparatively low temperature, and are there mixed with from 12 to 24 per cent. of oxygen gas. Oil gas made at low temperatures, per se, is of little use as an illuminant, as it burns with a smoky flame, and does not travel well, but when mixed with a certain amount of oxygen, it gives a very brilliant white light, and no smoke, while as far as experiments have at present gone, its traveling powers are much improved.
At first sight it seems a dangerous experiment to mix a heavy hydrocarbon gas with oxygen, but it must be remembered that although hydrogen and carbon monoxide only need to be mixed with half their own volume of oxygen to give a most explosive mixture, yet as the number of carbon and hydrogen atoms in the combustible gas increase, so does the amount of oxygen needed to give explosion. Thus coal gas needs rather more than its own volume, and ethylene three times its volume, to give the maximum explosive results, while these mixtures begin to be explosive when 10 per cent. of oxygen is mixed with hydrogen or water gas, 30 per cent. with coal gas, and over 50 per cent. of oil gas of the character used. It is claimed that if this gas was used as an enricher of coal gas, 5 per cent. of it would increase the luminosity of 16-candle gas by about 40 per cent.
Oxygen has been obtained for some time past from the air on a commercial scale by the Brin process, and at the present time there seems every prospect of our being able to obtain oxygen at a rate of about 3s. 6d. per 1,000 cubic feet. Another process by which this important result can also be obtained was first introduced by Tessie du Mothay, and has now just been revived. It consists of passing alternate currents of steam and air over sodic manganate heated to dull redness in an iron tube; the process has never been commercially successful, for the reason that the contents of the tube fused, and flowing over the surface of the iron rapidly destroyed the tubes or retorts, and also as soon as fusion took place, the mass became so dense that it had little or no action on the air passing over it. Now, however, this difficulty has been partly overcome by so preparing the manganate as to prevent fusion, and to keep it in a spongy state, which gives very high results, and the substance being practically everlasting, the cost of production is extremely low.
It is proposed to feed this by a separate system of pipes to small gas jets, and by converting them into practically oxyhydrogen blow pipes, to raise solid masses of refractory material to incandescence, and also by supplying oxygen in the same way to oil lamps of particular construction, to obtain a very great increase in illuminating power.
Whether these methods of employing cheap oxygen would be successful or not, I do not wish to discuss at the present time, but there is no doubt but that cheap oxygen would be an enormous boon to the gas manager, as by mixing 0.8 per cent. of oxygen with his coal gas before purification, he could not only utilize the method so successfully introduced by Mr. Valon at Ramsgate, but could also increase the illuminating value of his gas.
In speaking of the structure of flame, I pointed out that close to the burner from which the gas giving the flame is issuing, a space exists in which no combustion is going on—in other words, a flame is never in contact with the rim of the burner. This is best seen when the gas is turned low—with a batswing burner, for instance—turned so low that only a small non-luminous flame is left, the space between burner and flame will appear as great as the flame itself, while, if the gas is mixed with an inert diluent like carbon dioxide, the space can be very much increased.
Several theories have been brought forward to explain this phenomenon, but the true one is that the burner abstracts so much heat from the flame at that point that it is unable to burn there, and this can be proved by the fact that where a cold object touches the flame, a dividing space, similar to that noticed between flame and burner, will always be observed, and the colder the object and the more diluted the gas the greater is the observed space. If a cold metal wire or rod is held in a non-luminous flame, it causes an extinction of the gas for some considerable space around itself; but as the temperature of the rod rises, this space becomes smaller and smaller until the rod is heated to redness, and then the flame comes in contact with the rod.
In the same way, if the burner from which the gas is issuing be heated to redness, the space between burner and flame disappears. It has already been shown that cooling the flame by an inert diluent reduces the illuminating value, and finally renders it more luminous; and we are now in a position to discuss the points which should be aimed at in the construction of a good gas burner.
In the first place, a sensible diminution in light takes place when a metal burner is employed, and the larger the surface and thickness of the metal the worse will be its action on the illuminating power of the flame; but this cooling action is only influencing the bottom of the flame, so that with a small flame the total effect is very great, and with a very large flame almost nil.
The first point, therefore, to attend to is that the burner shall be made of a good non-conductor. In the next place, the flow of the gas must be regulated to the burner, as, if you have a pressure higher than that for which the burner is constructed, you at once obtain a roaring flame and a loss of illuminating power, as the too rapid rush of gas from the burner causes a mingling of gas and air and a consequent cooling of the flame. The tap also which regulates the flame is better at a distance from the burner than close to it, as any constriction near the burner causes eddies, which give an unsteady flame.
These general principles govern all burners, and we will now take the ordinary forms in detail. In the ordinary flat flame burner, given a good non-conducting material, and a well regulated gas supply, little more can be done, while burning it in the ordinary way, to increase its luminosity; and it is the large surface of flame exposed to the cooling action of the air which causes this form of burner to give the lowest service of any per cubic foot of gas consumed. Much is done, moreover, by faulty fittings and shades, to reduce the already poor light given out, because the light-yielding power of the flame largely depends upon its having a well rounded base and broad, luminous zone; and when a globe with a narrow opening is used with such a flame—as is done in 99 out of 100 cases—the updraught drags the flame out of shape, and seriously impairs its light-giving powers, a trouble which can be got over by having the globe with an opening at the bottom not less than 4 inches in diameter, and having small shoulders fixed to the burner, which draw out the flame and protect the base from the disturbing influence of draughts.
The Argand burner differs from the flat flame burners in that a circular flame is employed. The air supply is regulated by a cylindrical glass, and this form of burner gives a better service than the flat flame burner, as not only can the supply of gas and air be better adjusted, but the air being slightly warmed by the hot glass adds to the temperature of the flame, which is also increased by radiation from the opposite side of the flame itself.
The chief loss of light in such a burner depends upon the fact that, being circular, the light from the inner surface has to pass through the wall of flame, and careful photometric experiments show that the solid particles present in the flame so reduce its transparency that a loss amounting to about 25 per cent. of light takes place during its transmission.
The height of the flame also must be carefully adjusted to the size of the flame, as too long a chimney, by increasing the air supply unduly, cools, and so lowers the illuminating power of the flame. Experiments with carbureted water gas gave the following results, with a consumption of 5 cubic feet per hour:
| Size of Chimney. | Height of Flame. | Candle Power. | |
| 6 X 1-7/8 | 2-1/2 | 21 | |
| 7 X 1-7/8 | 2-1/4 | 21.3 | |
| 8 X 1-7/8 | 2-1/8 | 20.8 | |
| 9 X 1-7/8 | 1-7/8 | 18.2 |
For many years no advance was made upon these forms of burner, but when, ten years ago, it was recognized that anything which cools the flame reduces its value, while anything which increases its temperature raises its illuminating power, then a change took place in the forms of burner in use, and the regenerative burners, introduced by such men as Siemens, Grimston, and Bower, commenced what was really a revolution in gas lighting.
By utilizing the heat contained in the escaping products of combustion to raise the temperature of the gas and air which are to enter into combination in the flame, an enormous increase in the temperature of the solid particles of carbon in the flame is obtained, and a far greater and whiter light is the result.
The Bower lamp, in which (at any rate in the later forms) the flame burns between a downward and an upward current of air, was one of the first produced, and so well has it been kept up to date that it still holds its own; while as types of the "inverted cone" regenerative burner, we may also take the Cromarty and Wenham lights, which have been followed by a host of imitators, and so closely are the original types adhered to that one begins seriously to wonder what the use of the Patent Office really is.
The Schulke, and the last form of Siemens regenerative burner, however, stand apart from all the others by dealing with flat and not conical flames, and in both regeneration is carried on to a high degree. The only drawback to the regenerative burner is that it is by far the best form of gas stove as well as burner, and that the amount of heat thrown out by the radiant solid matter in the flame is, under some circumstances, an annoyance. But, on the other hand, we must not forget that this is the form best adapted for overhead burners, and that nearly every form of regenerative lamp can be adapted as a ventilating agent, and that with the withdrawal of the products of combustion from the air of the room, the great and only serious objection to gas as an illuminant disappears.
When coal gas is burned, the hydrogen is supposed to be entirely converted into water vapor, and the carbon to finally escape into the air as carbon dioxide; and if this were so, every cubic foot of gas consumed would produce approximately 0.52 cubic foot of carbon dioxide and 1.34 cubic feet of water vapor, while the illuminating power yielded by the cubic foot of gas will, of course, vary with the kind of burner used.
Roughly speaking, the ordinary types of burner give the following results:
| Products of Combustion per Candle Power. | |||
| Name of Burner. | Illuminating Power in Candles per c.f. of gas Consumed. | Carbon Dioxide. | Water Vapor. |
| Batswing. | 2.9 | 0.18 c.f. | 0.46 c.f. |
| Argand. | 3.3 | 0.16 c.f. | 0.40 c.f. |
| Regenerative. | 10.0 | 0.05 c.f. | 0.13 c.f. |
So that the regenerative forms of burner, by giving the greatest illuminating power per cubic foot of gas consumed, yield a smaller amount of vitiation to the air per candle of light emitted.
An ordinary room, say 16' X 12' X 10', would not be considered properly illuminated unless the light were at least equal to 32 candle power; and in the table below the amount of the oxygen used up and the products of combustion formed by each class of illuminant and burner in attaining this result are given, the number of adults who would exhale the same amount during respiration being also stated.
From these data it appears, according to rules by which the degree of vitiation of the air in any confined space is measured by the amount of oxygen used up and carbon dioxide formed, that candles are the worst offenders against health and comfort. Oil lamps come next, and gas least. This, however, is an assumption which practical experience does not bear out. Discomfort and oppression in a room lighted by candles or oil are less felt than in one lighted by any of the older forms of gas burner; and the partial explanation of this is to be found in the fact that, when a room is illuminated with candles or oil, people are contented with a feebler and more local light than when using gas. In a room of the size described, the inmates would be more likely to use two candles placed near their books, or on a table, than thirty-two scattered about the room.
Moreover, the amount of water vapor given off during the combustion of gas is greater than in the case of the other illuminants. Water vapor having a great power of absorbing radiant heat from the burning gas becomes heated, and diffusing itself about the room, causes great feeling of oppression; the air also being highly charged with moisture, is unable to take up so rapidly the water vapor which is always evaporating from the surface of our skin, whereby the functions of the body receive a slight check, resulting in a feeling of malaise.
Added to these, however, is a far more serious factor which has, up to the present, been overlooked, and that is that an ordinary gas flame, in burning, yields distinct quantities of carbon monoxide and acetylene, the prolonged breathing of which in the smallest traces produces headache and general physical discomfort, while its effect upon plant life is equally marked.
AMOUNT OF OXYGEN REMOVED FROM THE AIR, AND CARBON DIOXIDE AND WATER VAPOR GENERATED
TO GIVE AN ILLUMINATION EQUAL TO 32 CANDLE POWER.
| (The amount of light required in a room 16' X 12' x 10'.) | ||||||
| Products of Combustion | ||||||
| Illuminant | Quantity of Materials Used | Oxygen Removed | Water Vapor | Carbon Dioxide | Adults | |
| Sperm Candles | 3,840 grains | 19.27 c.f. | 13.12 c.f. | 13.12 c.f. | 21.8 | |
| Paraffin Oil | 1,984 grains | 12.48 c.f. | 7.04 c.f. | 8.96 c.f. | 14.9 | |
| Gas (London)-- | ||||||
| Burners: | ||||||
| Batswing | 11 c.f. | 13.06 c.f. | 14.72 c.f. | 5.76 c.f. | 9.6 | |
| Argand | 9.7 c.f. | 11.52 c.f. | 12.80 c.f. | 5.12 c.f. | 8.5 | |
| Regenerative | 3.2 c.f. | 3.68 c.f. | 4.16 c.f. | 1.60 c.f. | 2.6 | |
Ever since the structure of flame has been noted and discussed, it has been accepted as a fact beyond dispute that the outer almost invisible zone which is interposed between the air and the luminous zone of the flame is the area of complete combustion, and that here the unburnt remnants of the flame gases, meeting the air, freely take up oxygen and are converted into the comparatively harmless products of combustion, carbon dioxide and water vapor, which only need partial removal by any haphazard process of ventilation to keep the air of the room fit to support animal life. I have, however, long doubted this fact, and at length, by a delicate process of analysis have been able to confirm my suspicions. The outer zone of a luminous flame is not the zone of complete combustion; it is a zone in which luminosity is destroyed in exactly the same way that it is destroyed in the Bunsen burner; that is the air penetrating the flame so dilutes and cools down the outer layer of incandescent gas that it is rendered non-luminous, while some of the gas sinks below the point at which it is capable of burning, with the result that considerable quantities of the products of incomplete combustion carbon monoxide and acetylene escape into the air, and render it actively injurious.
I have proved this by taking a small platinum pipe, with a circular loop on the end, the interior of the loop being pierced with minute holes, and by making a circular flame burn within the loop so that the non-luminous zone of the flame just touched the inside of the loop, and then by aspiration so gentle as not to distort the shape of the flame, withdrawing the gases escaping from the outer zone. On analyzing these by a delicate process, which will be described elsewhere, I arrived at the following results:
GASES ESCAPING FROM THE OUTER ZONE OF FLAME.
| Luminous. | Bunsen. | |
| Nitrogen. | 76.612 | 80.242 |
| Water vapor. | 14.702 | 13.345 |
| Carbon dioxide. | 2.201 | 4.966 |
| Carbon monoxide. | 1.189 | 0.006 |
| Oxygen. | 2.300 | 1.430 |
| Marsh gas. | 0.072 | 0.003 |
| Hydrogen. | 2.888 | 0.008 |
| Acetylene. | 0.036 | Nil. |
| 100.000 | 100.000 |
The gases leaving the luminous flame show that the diluting action of the nitrogen is so great that considerable quantities even of the highly inflammable and rapidly burning hydrogen escape combustion, while the products of incomplete combustion are present in sufficient quantity to account perfectly for the deleterious effects of gas burners in ill-ventilated rooms. The analyses also bring out very clearly the fact that, although the dilution of coal gas by air in atmospheric burners is sufficient to prevent the decomposition of the heavy hydrocarbons with liberation of carbon, and so destroy luminosity, yet the presence of the extra supply of oxygen does make the combustion far more perfect, so that the products of incomplete combustion are hardly to be found in the escaping gases.
These experiments are of the gravest import, as they show more clearly than has ever been done before the absolute necessity for special and perfect ventilation where coal gas is employed for the illumination of our dwelling rooms.
When coal gas was first employed during the early part of this century as an illuminating agent, the low pitch of the old fashioned rooms, and the excess of impurities in the gas, rendered it imperative that the products of combustion of the sulphur-laden gas should be conducted from the apartment, and for this purpose arrangements of tubes with funnel shaped openings were suspended over the burners. The noxious gases were thus conveyed either to the flue or open air; but this type of ventilator was unsightly in the extreme, and some few attempts were made to replace it by a more elegant arrangement, as in the ventilating lamp invented by Faraday, and in the adaptation of the same principle by Mr. I.O.N. Rutter, who strove for many years to direct attention to the necessity of removing the products of combustion from the room. But with the increase of the gas industry, the methods for purifying the coal gas became gradually more and more perfect, while the rooms in the modern houses were made more lofty; and the products of combustion being mixed with a larger volume of air, and not containing so many deleterious constituents, became, if not much less noxious, at all events less perceptible to the nose. As soon as this point was reached, the ventilating tubes were discarded, and from that day to this the air of our dwelling rooms has been contaminated by illuminants, with hardly an effort to alleviate the effect produced upon health. I say "hardly an effort," for the Messrs. Boyle tried, by their concentric tube ventilators, to meet the difficulty, while Mr. De la Garde and Mr. Hammond have each constructed lamps more or less on the principle of the Rutter lamp; but either from their being somewhat unsightly, or from their diminishing the amount of light given out, none of them have met with any degree of success. In places of public entertainment, where large quantities of coal gas are consumed for illuminating purposes, the absolute necessity for special ventilation gave rise to the "sun burner," with its ventilating shaft. This, however, gives but a very poor illuminating power per cubic foot of gas consumed, due partly to the cooling of the flame by the current of air produced, and partly to its distance from the objects to be illuminated.
The great difficulty which in the whole history of ventilation has opposed itself to the adoption of proper arrangements for removing the products of combustion has been the necessity of bringing the tube to carry off the gases low down into the room, and of incasing the burner in such a way that none of the products should escape; but with the present revolution in gas burners this necessity is entirely done away with, and the regenerative burner offers the means not only of removing all the products of combustion but also of effecting thorough ventilation of the room itself, as experiments made some few years ago showed me that a ventilating regenerative burner, burning 20 cubic feet of gas per hour and properly fitted, will not only remove all its own products of combustion, but also over 5,000 cubic feet per hour of the vitiated air from the upper part of the room. I am quite aware that many regenerative lamp makers raise various objections to fitting ventilating lamps, these being chiefly due to the fact that it requires considerable trouble to fit them properly; but I think I have said enough to show the absolute necessity of some such system, and when there is a general demand for ventilating lamps, engineering skill will soon find means to overcome any slight difficulties which exist.
Having disposed in a few words of a subject which, if fully treated, would occupy a long course of lectures by itself, I will pass on to the consideration of gas as at present used as a fuel.
There is no doubt that gas is the most convenient and in many ways one of the best forms of fuel for heating and cooking purposes, and the efforts which all large gas companies are now making to popularize and increase the use of gas for such purposes will undoubtedly bear fruit in the future. But before the day can come for gas to be used in this way on a large scale, there is one fact which the gas manager and gas stove manufacturer must clearly realize and submit to, and that is that no gas stove or gas water heater, of any construction, should be sent out or fitted without just as great care being taken to provide for the carrying away of the products of combustion as if an ordinary fuel range was being fitted. Do not for one moment allow yourself to be persuaded that, because a gas stove or geyser does not send out a mass of black smoke, the products of combustion can be neglected and with safety allowed to mingle with the atmosphere we are to breathe.
Scarcely a winter passes but one or more deaths are recorded from the products of combustion given off from various forms of water heaters used in bath rooms; scarcely a cookery class is given, with gas stoves, that one or more ladies do not have to leave suffering from an intense headache, and often in an almost fainting condition. And the same cause which brings about these extreme cases, on a smaller scale causes such physical discomfort to many delicately organized persons that a large class exist who absolutely and resolutely decline to have gas as an illuminant or fuel in any of their living rooms; and if the use of gas, more especially as fuel, is to be extended, and if gas is to hold its own in the future against such rivals as the electric light, then those interested in gas and gas stoves must face the problem, and by improving the methods of burning and using gas do away with the present serious drawbacks which exist to its use.
The feeling has gradually been gaining ground in the public mind that, when atmospheric burners and other devices for burning coal gas are employed for heating purposes, certain deleterious products of incomplete combustion find their way into the air, and that this takes place to a considerable extent is shown by the facts brought forward in a paper read by Mr. William Thomson before the last meeting of the British Association.
Mr. Thomson attempted to separate and determine the quantity of carbon monoxide and hydrocarbons present in the flue gases from various forms of gas stoves and burners, but, like every other observer who has attempted to solve this most difficult problem, he found it so beset with difficulties that he had to abandon it, and contented himself with determining the total amounts of carbon and hydrogen escaping in an unburned condition, experiments which showed that the combustion of gas in stoves for heating purposes is much more incomplete than one had been in the habit of supposing, but his experiments give no clew as to whether the incompletely burned matter consisted of such deleterious gases as carbon monoxide and acetylene, or comparatively harmless gases, such as marsh gas and hydrogen. After considerable work upon the subject, I have succeeded in doing this by a very delicate process of analysis, and I now wish to lay some of my results before you.
If a cold substance, metal or non-metal, be placed in a flame, whether it be luminous or non-luminous, it will be observed that there is a clear space, in which no combustion is taking place, formed round the cool surface, and that as the body gets heated so this space gets less and less until, when the substance is at the same temperature as the flame itself, there is contact between the two. Moreover, when a luminous flame is employed in this experiment the space still exists between the cool body and the flame, but you also notice that the luminosity is decreased over a still larger area although the flame exists.
This meaning that, in immediate contact with the cold body, the temperature is so reduced that the flame cannot exist, and so is extinguished over a small area; while over a still larger space the temperature is so reduced that it is not hot enough to bring about decomposition of the heavy hydrocarbons with liberation of carbon to the same extent as in hotter portions of the flame. Now, inasmuch as when water is heated or boiled in an open vessel, the temperature cannot rise above 100°C., and as the temperature of an ordinary flame is over 1,000°C., it is evident that the burning gas can never be in contact with the bottom of the vessel, or, in other words, the gas is put out before combustion is completed, and the unburned gas and products of incomplete combustion find their way into the air and render it perfectly unfit for respiration.
The portion of the flame which is supposed to be the hottest is about half an inch above the tip of the inner zone of the flame, and it is at this point that most vessels containing water to be heated are made to impinge on the flame; and it is this portion of the flame, also, which is utilized for raising various solids to a temperature at which they radiate heat.
In order to gain an insight into the amount of contamination which the air undergoes when a geyser or cooking stove is at work, I have determined the composition of the products of combustion, and the unburned gases escaping when a vessel containing water at the ordinary temperatures is heated up to the boiling point by a gas flame, the vessel being placed, in the first case, half an inch above the inner cone of the flame, and in the second, at the extreme outer tip of the flame.
GASES ESCAPING DURING CHECKED COMBUSTION.
| Bunsen flame. | Luminous flame. | |||
| Inner. | Outer. | Inner. | Outer. | |
| Nitrogen | 75.75 | 79.17 | 77.52 | 69.41 |
| Water vapor | 13.47 | 14.29 | 11.80 | 19.24 |
| Carbon dioxide | 2.99 | 5.13 | 4.93 | 8.38 |
| Carbon monoxide | 3.69 | Nil. | 2.45 | 2.58 |
| Marsh gas | 0.51 | 0.31 | 0.95 | 0.39 |
| Acetylene | 0.04 | Nil. | 0.27 | Nil. |
| Hydrogen | 3.55 | 0.47 | 2.08 | Nil. |
| 100.00 | 100.00 | 100.00 | 100.00 | |
These figures are of the greatest interest, as they show conclusively that the extreme top of the Bunsen flame is the only portion of the flame which can be used for heating a solid substance without liberating deleterious gases; and this corroborates the previous experiment on the gases in the outer zone of a flame, which showed that the outer zone of a Bunsen flame is the only place where complete combustion is approached.
Moreover, this sets at rest a question which has been over and over again under discussion, and that is whether it is better to use a luminous or a non-luminous flame for heating purposes. Using a luminous flame, it is impossible to prevent a deposit of carbon, which is kept by the flame at a red heat on its outer surface, and the carbon dioxide formed by the complete combustion of the carbon already burned up in flame is reduced by this back to carbon monoxide, so that even in the extreme tip of a luminous flame it is impossible to heat a cool body without giving rise to carbon monoxide, although acetylene being absent, gas stoves, in which small flat flame burners are used, have not that subtile and penetrating odor which marks the ordinary atmospheric burner stove, with the combustion checked just at the right spot for the formation of the greatest volume of noxious products.
It is the contact of the body to be heated with the flame before combustion is complete which gives rise to the greatest mischief; any cooling of the flame extinguishes a portion of the flame, and the gases present in the flame at the moment of extinction creep along the cooled surface and escape combustion.
Dr. Blochmann has shown the composition of the gases in various parts of the Bunsen flame to be as follows:
| Height above tube. | In tube. | 1 inch. | 2 inch. | 3 inch. | Complete |
| Air with 100 vols. gas | 253.9 | 284.7 | 284.5 | 484.3 | 608.8 |
| Hydrogen | 48.6 | 36.4 | 17.7 | 16.1 | Nil. |
| Marsh gas | 39.0 | 40.1 | 28.0 | 5.7 | Nil. |
| Carbon monoxide | 2.9 | 2.2 | 19.9 | 12.7 | Nil. |
| Olefiant gas | 4.0 | 3.4 | 2.2 | Nil. | Nil. |
| Buteylene | 3.0 | 2.5 | 1.6 | Nil. | Nil. |
| Oxygen | 52.7 | 52.0 | 21.7 | Nil. | Nil. |
| Nitrogen | 199.1 | 223.8 | 225.9 | 382.4 | 482.3 |
| Carbon dioxide | 0.8 | 3.5 | 13.0 | 41.7 | 62.4 |
| Water vapor | 3.1 | 11.8 | 45.8 | 116.1 | 141.2 |
Which results show that it would be impossible to check the flame anywhere short of the extreme tip (where complete combustion is approximately taking place), without liberating deleterious products. I think I have said enough to show that no gas stove, geyser or gas cooking stove should be used without ample and thorough means of ventilation being provided, and no trace of the products of combustion should be allowed to escape into the air; until this is done, the use of improper forms of stoves will continue to inflict serious injury on the health of the people using them, and this will gradually result in the abandonment of gas as a fuel, instead of, as should be the case, its coming into general use. The English householder is far too prone to accept what is offered to him, without using his own common sense, and will buy the article which tickles his eye the most and his pocket the least, on the bare assurance of the shopkeeper, who is only anxious to sell; but when he finds that health and comfort are in jeopardy, and has discarded the gas stove, it will take years of labor to convince him that it was the misuse of gas which caused the trouble. Already signs are not wanting that the employers of gas stoves are beginning to fight shy of them, and I earnestly hope that the gas managers of the kingdom will bring pressure to bear upon the stove manufacturers to give proper attention to this all important question.
So strongly do I feel the importance of this question to the gas world and the public, that I freely offer to analyze the products of combustion given off by any gas stove or water heater sent to me at Greenwich during the next six months, on one condition, and that is that the results, good, bad, or indifferent, will be published in a paper before this Society, which has always been in the front when matters of great sanitary importance to the public had to be taken up. And if after that the public like to buy forms of apparatus which have not been certified, it is their own fault; but I do think that the maker of any stove or geyser which causes a death should be put upon his trial for manslaughter.
In conclusion, let us consider for a moment what is likely to be the future of gas during the next half century. The labor troubles, bad as they are and have been, will not cease for many a weary year. The victims of imperfect education (more dangerous than none at all, as, while destroying natural instinct, it leaves nothing in its place) will still listen and be led by the baneful influence of irresponsible demagogues, who care for naught so long as they can read their own inflammatory utterances in the local press, and gain a temporary notoriety at the expense of the poor fools whose cause they profess to serve. The natural tendency of this will be that every labor-saving contrivance that can will be pressed into the gas manager's service; and that, although coal (of a poorer class than at present used) will still be employed as a source of gas, the present retort setting will quickly give way to inclined retorts on the Coze principle; while, instead of the present wasteful method of quenching the red hot coke, it will be shot direct into the generator of the water gas plant, and the water gas carbureted with the benzene hydrocarbons derived from the smoke of the blast furnace and coke oven, or from the creosote oil of the tar distiller, by the process foreshadowed in the concluding sentences of my last lecture. It will then be mixed with the gas from the retorts, and will supply a far higher illuminant than we at present possess. In parts of the United Kingdom, such as South Wales, where gas coal is dear, and anthracite and bastard coals are cheap, water gas highly carbureted will entirely supplant coal gas, with a saving of fifty per cent. on the prices now existing in those districts. While these changes have been going on, and while improved methods of manufacture have been tending to the cheapening of gas, it will have been steadily growing in public favor as a fuel; and if in years to come the generation of electricity should have been so cheapened as to allow it to successfully compete with gas as an illuminant, the gas works will still be found as busy as of yore, the holder of gas shares as contented as to-day; for with a desire for a purer atmosphere and a white mist instead of a yellow fog, gas will have largely supplanted coal as a fuel, and gas stoves, properly ventilated and free from the reproaches I have hurled at them to-night, will burn a gas far higher in its heating power, far better in its power of bearing illuminating hydrocarbons, and free from poisonous constituents.
When the demand for it arises, hydrogen gas can be made as cheaply as water gas itself, and when time is ripe for a fuel gas for use in the house, it is hydrogen and not water gas which will form its basis. With carbureted water gas and 20 per cent. of carbon monoxide we are still below the limit of danger, but a pure water gas with over 40 per cent. of the same insidious element of danger will never be tolerated in our households. Already a patent has been taken by Messrs. Crookes and Ricarde-Seaver for purifying water gas from carbon monoxide, and converting it mainly into hydrogen by passing it at a high temperature through a mixture of lime and soda lime, a process which is chemically perfect, as the most expensive portion of the material used could be recovered; but in the present state of the labor market it is not practical, as for the making of every 100,000 cubic feet of gas, fifteen tons of material would have to be handled, the cost of labor alone being sufficient to prevent its being adopted; moreover, hydrogen can be made far cheaper directly.
From the earliest days of gas making, the manufacture of hydrogen by the passage of steam over red-hot iron has been over and over again mooted, and attempted on a large scale, but several factors have combined to render it futile.
In the first place, for every 478.5 cubic feet of hydrogen made under perfect theoretical conditions never likely to be obtained in practice, 56 lb. of iron were converted into the magnetic oxide, and as there was no ready sale for this article, this alone would prevent its being used as a cheap source of hydrogen; the next point was that when steam was passed over the red-hot iron, the temperature was so rapidly lowered that the generation of gas could only go on for a very short period, while, finally, the swelling of the mass in the retort and fusion of some of the magnetic oxide into the side renders the removal of the spent material almost an impossibility. These difficulties can, however, be got over. Take a fire clay retort, six feet long and a foot in diameter, and cap it with a casting bearing two outlet tubes closed by screw valves, while a similar tube leads from the bottom of the retort. Inclose this retort by a furnace chamber of iron lined with fire brick, leaving a space of two feet six inches round the retort, and connect the top of the furnace chamber with one opening at the top of the upright retort, while air blasts lead into the bottom of the furnace chamber, below rocking fire bars, which start at bottom of the retort, and slope upward, to leave room for ash holes closed by gas tight covers. The retort is filled with iron or steel borings, alone if pure hydrogen is required, or cast into balls with pitch if a little carbon monoxide is not a drawback, as in foundry work. The furnace chamber is now filled with coke, fed in through manholes, or hoppers, in the top, and the fuel being ignited, the blast is turned on, and the mixture of nitrogen and carbon monoxide passes over the iron, heating it to a red heat, while the fuel in contact with the retort does the same thing.
When the fuel and retort full of iron are at a cherry-red heat, the air blast is cut off, and the pipe connecting the furnace and retort, together with the pipe in connection with the bottom of the retort, are closed, and steam, superheated by passing through a pipe led round the retort or interior wall of the furnace, is injected at the bottom of the red-hot mass of iron, which decomposes it, forming magnetic oxide of iron and hydrogen, which escapes by the second tube at the top of the retort, and is led away either to a carbureting chamber if required for illumination, or direct to the gasholder if wanted as a fuel. The mass of incandescent fuel in the furnace chamber, surrounding the retort, keeping up the temperature of retort and iron sufficiently long to enable the decomposition to be completed.
The hydrogen and steam valves are now closed and the air blast turned on. The hot carbon monoxide passing over the hot magnetic oxide quickly reduces it down to metallic iron, which, being in a spongy condition, acts more freely on the steam during later makes than it did at first, and being infusible at the temperature employed, may be used for a practically unlimited period.
What more simple method than this could be desired? Here we have the formation of the most valuable of all fuel gases at the cost of the coke and steam used, a gas also which has double the carrying power for hydrocarbon vapors possessed by coal gas, while its combustion gives rise to nothing but water vapor.
In this course of lectures I have left much unsaid and undone which I should have liked to have had time to accomplish, and if I have been obliged to leave out of consideration many important points, it is the time at my disposal and not my will which is to blame. And now, in conclusion, I wish to express my thanks to my assistants, Messrs. J.A. Foster and J.B. Warden, who have heartily co-operated with me in much of the work embodied in these lectures.
The celebrated philosopher Bacon, the founder of the experimental method, claimed that we see better with one eye than with two, because the attention is more concentrated and becomes profounder. "On looking in a mirror," says he, "we may observe that, if we shut one eye, the pupil of the other dilates." To this question: "But why, then, have we two eyes?" he responds: "In order that one may remain if the other gets injured." Despite the reasoning of the learned philosopher, we may be permitted to believe that the reason that we have two eyes is for seeing better and especially for perceiving the effects of perspective and the relief of objects. We have no intention of setting forth here the theory of binocular vision; one simple experiment will permit any one to see that the real place of an object is poorly estimated with one eye. Seated before a desk, pen in hand, suddenly close one eye, and, at the same time, stretch out the arm in order to dip the pen in the inkstand; you will fail nine times out of ten. It is not in one day that the effects of binocular vision have been established, for the ancients made many observations on the subject. It was in 1593 that the celebrated Italian physicist Porta was the first to give an accurate figure of two images seen by each eye separately, but he desired no apparatus that permitted of reconstituting the relief on looking at them. Those savants who, after him, occupied themselves with the question, treated it no further than from a theoretical point of view. It was not till 1838 that the English physicist Wheatstone constructed the first stereoscopic apparatus permitting of seeing the relief on examining simultaneously with each of the eyes two different images of an object, one having the perspective that the right eye perceives, and the other that the left eye perceives.
This apparatus is described in almost all treatises on physics. We may merely recall the fact that it operated by reflection, that is to say, the two images were seen through the intermedium of two mirrors making an angle of 45 degrees. The instrument was very cumbersome and not very practical. Another English physicist, David Brewster, in 1844 devised the stereoscope that we all know; but, what is a curious thing, he could not succeed in having it constructed in England, where it was not at first appreciated. It was not till 1850 that he brought it to Paris, where it was constructed by Mr. Soleil and his son-in-law Duboscq. Abbot Moigno and the two celebrated opticians succeeded, not without some difficulty, in having it examined by the official savants; but, at the great exposition of 1851, it was remarked by the Queen of England, and from this moment Messrs. Soleil & Duboscq succeeded with difficulty only in satisfying the numerous orders that came from all parts. As photography permitted of easily making identical images, but with different perspective, it contributed greatly to the dissemination of the apparatus.
The stereoscope, such as we know it, presents the inconvenience of being incapable of being used by but one person at once. Several inventors have endeavored to render the stereoscopic images visible to several spectators at the same time. In 1858, Mr. Claudet conceived the idea of projecting the two stereoscopic images upon ground glass in superposing them. The relief was seen, it appears, but we cannot very well explain why; the idea, however, had no outcome, because the image, being quite small, could be observed by but three or four persons at once. It was Mr. D'Almeida, a French physicist, who toward the same epoch solved the problem in a most admirable manner, and we cannot explain why his process (that required no special apparatus) fell into the desuetude from which Mr. Molteni has just rescued it and obtained much success.
This is in what it consists: The impression of the relief appears when each eye sees that one of the two images which presents the perspective that it would perceive if it saw the real object. If we take two transparent stereoscopic images and place each of them in a projection lantern, in such a way that they can be superposed upon the screen, we shall obtain thereby a single image. It will always be a little light and soft, as the superposition cannot be effected accurately, the perspective not being the same for each of them. It is a question now to make each eye see the one of the two images proper to it. To this effect, Mr. D'Almeida conceived the very ingenious idea of placing green glass in the lantern in front of the image having the perspective of the right eye, and a red glass in front of the other image. As green and red are complementary colors, the result was not changed upon the screen; there was a little less light, that was all. But if, at this moment, the spectator places a green glass before his right eye and a red one before his left, he will find himself in the condition desired for realizing the effect sought.
Each eye will then see only the image responding to the coloration chosen, and, as it is precisely the one which has the perspective proper to it, the relief appears immediately. The effect is striking. We perceive a diffused image upon the screen with the naked eye, but as soon as we use one special eye-glass the relief appears with as much distinctness as in the best stereoscope. One must not, for example, reverse his eye-glass, for if (things being arranged as we have said) he looks through a red glass before his right eye, and through a green one before his left, it is the image carrying the perspective designed for the right eye that will be seen by the left eye, and reciprocally. There is then produced, especially with certain images, a very curious effect of reversed perspective, the background coming to the front.
Now that photography is within every one's reach, and that many amateurs are making stereopticon views and own projection lanterns, we are persuaded that the experiment will be much more successful than it formerly was. An assemblage of persons all provided with colored eye-glasses is quite curious to contemplate. Our engraving represents a stereopticon seance, and the draughtsman has well rendered the effect of the two luminous and differently colored fascicles superposed upon the screen.
In a preceding note upon the same subject, Mr. Hospitalier remarked that upon combining these effects of perspective with those of the praxinoscope, which give the sensation of motion, we would obtain entirely new effects. It would be perhaps complicated as to the installation, and especially as to the making of the images, but, in certain special cases (for giving the effect of a machine in motion, for example), it might render genuine services.—La Nature.
On July 2, 1889, ten Plymouth Rock hens, one year old, and as nearly as possible of uniform size, were selected from a flock of thirty-five. At the same time ten chickens, hatched from the same hens mated with a Plymouth Rock cock, were similarly chosen. The chickens were about six weeks old, healthy and vigorous and of nearly the same size. Up to the time of purchase both hens and chickens had full run of the farm. The hens foraged for themselves and were given no food; the chickens had been fed corn meal dough, sour milk and table scraps.
A preliminary feeding trial was continued for twenty-five days, during which time both hens and chickens were confined, all together, in a fairly well lighted and ventilated room, and fed a great variety of food, in order that all should go into the feeding trial as nearly as possible in the same condition. During this preliminary feeding both hens and chickens increased in live weight. The ten hens from a total of 44 lb. 12 oz. to 47 lb. 1.5 oz., or 3.75 oz. each, and laid 93 eggs. The chickens from a total of 9 lb. 15 oz. to 18 lb., or 12.9 oz. each.
Food, shells and water were kept constantly before the fowls. Basins which contained the food and water were kept within a box constructed of lath, so arranged that the fowls could reach between the slats and procure food and drink without wasting or soiling.
July 26th the hens and chickens were each separated into two lots of five each, as follows:
Hens, nitrogenous ration, weighed 23 lb. 8.5 oz.
Hens, carbonaceous ration, weighed 23 lb. 9 oz.
Chickens, nitrogenous ration, weighed 8 lb. 15 oz.
Chickens, carbonaceous ration, weighed 9 lb. 1 oz.
The four lots were placed in separate pens where they remained during the entire experiment, which lasted 125 days. They were fed and watered once daily, and an account kept of the food eaten and water drank. At each feeding the food and water remaining were weighed back and deducted from the amount charged at the previous feeding.
The hens and chickens fed a nitrogenous ration were given daily all they would eat of the following mixture: 1/3 part wheat bran, 1/3 part wheat shorts, 1/3 part cotton seed meal, 2 parts skimmed milk, and will be designated Lot I.
The hens and chickens fed a carbonaceous ration were given daily all they would eat of a ration of cracked maize and maize dough, and will be designated Lot II.
Both groups were given a small amount of green clover as long as it lasted, and afterward cabbage.
For convenience the experiment was divided into five periods of twenty five days.
During the first period all the fowls seemed in good health except the carbonaceous fed chickens; they, during this as in all succeeding periods, were restless and peevish, always moping or hunting for something to eat, though their trough was filled. When fed they would greedily take a few mouthfuls and then, with their hunger still unappeased, would leave the dish. They always ate ravenously the green food which was given them, as did the hens and chickens of Lot I. The hens of Lot II., on the contrary, seemed quite willing to squat about the pen and subsist on the maize diet, and strangely enough cared little for green food. The clear maize diet was accompanied by such ill effects that the chickens of each lot, after the first period, were given daily each one-fourth ounce of wheat, and the hens each one ounce. The wheat was increased during the fourth and fifth periods in the case of the chickens to one ounce each. During the second period one of the chickens fed nitrogenous food, and during the third period another of the same lot were taken ill and removed from the experiment. Both seemed to be suffering from impacted crops, as the stomach and gizzard in each case were found to be empty.
The fact that the sick chickens disliked the nitrogenous ration, and since the first period the amount of food eaten by the hens and chickens of Lot I had continually decreased, led to the belief that their food might be too nitrogenous, and as during the last days of the third period one of the hens in Lot I was also ill, it was decided to discontinue the use of cotton seed meal and to use linseed meal instead. The hen recovered soon after the change in food.
The supply of skim milk running short in the last two periods, water was used instead in mixing the ration of the lots fed nitrogenous food.
At the beginning of the fifth period one-half of the linseed meal in the ration of Lot I was removed, and cotton seed meal substituted. This combination seemed a happy one, for on this ration both hens and chickens made large gains.
At the end of the experiment little difference could be seen in the hens of the two groups; but the two lots of chickens were in striking contrast. While the chickens fed on nitrogenous food were large, plump, healthy, active, and well feathered, the chickens fed on a carbonaceous ration were in general much smaller, sickly, and in several cases almost destitute of feathers. Two of them had perfectly bare backs, and so ravenous were they for flesh and blood that they began eating one another.
The inability of the chickens fed on a carbonaceous diet to throw out new feathers and the ability of the chickens fed on a nitrogenous diet to grow an enormous coat of feathers is a splendid illustration of the effect of the composition of the food in supplying certain requirements of animal growth. It was plain to see that maize, even when assisted by a small amount of wheat and green clover, could not supply sufficient nitrogen for the growth of feathers.
It will thus be seen that while both lots of hens lost weight during the experiment, the loss was slightly greater with those fed nitrogenous food, but these produced by far the most eggs.
The chickens fed on nitrogenous food just about doubled in weight, while those fed carbonaceous food only added about one-third to their weight.
During the first week the carbonaceous fed hens laid three eggs while the others laid two. The two groups were, therefore, practically evenly divided at the start as to the condition of the laying stage. At the end of the first period the nitrogenous fed hens had laid forty-three eggs and the carbonaceous fed hens had laid twenty. During the next twenty-five days the former laid thirty and the latter six; during the third period the former laid six and the latter not any. From this time on no eggs were received from either group. The decline in egg production was probably due in large part to the fact that the hens began to moult during the second period, and continued to do so during the rest of the experiment.
The eggs laid by the nitrogenous fed hens were of small size, having a disagreeable flavor and smell, watery albumen, an especially small, dark colored yolk, with a tender vitelline membrane, which turned black after being kept several weeks. While the eggs of the carbonaceous fed hens were large, of fine flavor, of natural smell, large normal albumen, an especially large, rich yellow yolk, with strong vitelline membrane, which was perfectly preserved after being kept for weeks in the same brine with the other eggs.
TOTAL FOOD CONSUMED DURING EXPERIMENT.
| Lot. I.--Nitrogenous. | Lot. II.--Carbonaceous. | ||||
| Hens. | *Chicks | Hens. | Chicks. | ||
| lb. | lb. | lb. | lb. | ||
| Bran. | 29.90 | 21.85 | Maize. | 82.15 | 51.30 |
| Shorts. | 29.90 | 21.85 | Green clover. | 18.75 | 18.75 |
| Cotton seed meal. | 21.48 | 13.24 | Cabbage. | 16.00 | 16.00 |
| Linseed meal. | 8.43 | 8.61 | Wheat | 15.63 | 11.71 |
| Skimmed milk. | 105.49 | 61.33 | |||
| Wheat. | 15.63 | 11.71 | |||
| Green clover. | 18.75 | 18.75 | |||
| Cabbage. | 16.00 | 16.00 | |||
| Total. | 245.58 | 173.34 | Total. | 132.53 | 92.76 |
| Nutritive ratio. | 1:3.1 | 1:3 | Nutritive ratio. | 1:7.8 | 1:8 |
* Calculated for five chicks, based upon the amount eaten by the three after the two sick were removed.
EGGS LAID AND GAIN IN WEIGHT--HENS.
| Lot I. | Lot II. | ||
| Nitrogenous. | Carbonaceous. | ||
| Live weight, July 26. | 23.53 | 23.56 | |
| Live weight, November 27. | 21.31 | 22.00 | |
| Loss. | 2.22 | 1.56 | |
| Number of eggs laid. | 79.00 | 26.00 | |
| Weight of eggs laid lb. | 8.25 | 2.92 | |
| Average weight of eggs, oz. | 1.67 | 1.80 | |
| Gain in weight, including eggs, lb. | 6.03 | 1.36 |
GAIN IN LIVE WEIGHT--CHICKENS.
| Lot I. | Lot II. | ||
| Nitrogenous. | Carbonaceous. | ||
| Live weight, July 26. | 8.94 | 9.06 | |
| Live weight, November 27. | 17.89 | 12.63 | |
| Gain, lb. | 8.95 | 3.57 | |
| Gain, per cent. | 100.11 | 39.40 |
Samples of the eggs from each lot of fowls were privately marked and sold to a boarding house where the cook did not know that the eggs were undergoing a test. On meeting the cook several days later the following words were heard: "Do you expect me to cook such eggs as these! About every other one is spoiled." On examination of the ovaries after slaughtering, it was found that in the case of one of the carbonaceous fed hens the ovules were in a more advanced stage, but on the whole the nitrogenous fed hens were much nearer the laying period. With this single exception, the clusters of ovules in the carbonaceous fed hens were uniformly small. Neither group would have laid under any probability for several weeks. It would seem from these facts, together with the fact that during the experiment the nitrogenous fed hens laid more than three times as many eggs, that a nitrogenous ration stimulates egg production.
On November 27 the fowls were slaughtered. Each fowl was weighed, wrapped in a bag to prevent floundering, and killed by severing an artery in the roof of the mouth. The blood was caught in a glass jar. The fowls were then picked and the feathers weighed, after which the body was laid open longitudinally by cutting alongside the sternum and through the back bone. When all had been thus prepared, they were hung up in groups to be photographed, but the photographs were quite unsatisfactory so far as showing the relative proportions of fat and lean. The accompanying drawing made from the photograph shows the relative development of an average pair of chickens. Attention is particularly called to the thighs.
One-half of each fowl was tested by cooking for flavor, succulence, and tenderness. The other half was carefully prepared for chemical analysis by separating the meat from the bones. The flesh was thoroughly mixed and run through a sausage cutter, mixed again, and the process repeated three times. From different parts of this mixture a large sample was taken, from which the chemist took his samples for analysis. The right tibia of each fowl was tested for strength by placing it across two parallel bars and suspending a wire on its center, on which were placed small weights until the bone gave way.
DRESSED WEIGHT, INTERNAL ORGANS, ETC.
| Hens. | Chickens. | |||
| Lot I. | Lot II. | Lot I. | Lot II. | |
| Nitrogenous. | Carbonaceous. | Nitrogenous. | Carbonaceous. | |
| lb. | lb. | lb. | lb. | |
| Live weight | 21.31 | 22.0 | 17.89 | 12.63 |
| Dressed weight. | 14.86 | 15.09 | 12.01 | 8.89 |
| Dressed weight, per cent. | 69.7 | 68.6 | 67.1 | 70.5 |
| Weight of blood. | 0.75 | 0.66 | 0.55 | 0.34 |
| Weight of feathers. | 1.41 | 1.25 | 1.28 | 0.66 |
| Weight of intestinal fat. | 0.59 | 1.98 | 0.34 | 0.66 |
| Weight of offal. | 3.70 | 3.02 | 3.62 | 2.08 |
| Weight of bones. | 3.47 | 3.63 | 3.18 | 2.69 |
| Weight of flesh. | 11.39 | 11.47 | 8.93 | 6.20 |
The breaking strain of the right tibia was as follows for the hens and chickens of the various lots:
| Average hens, | nitrogenous. | 48.16 |
| " | carbonaceous. | 51.74 |
| Average chickens, | nitrogenous. | 46.64 |
| " | carbonaceous. | 31.18 |
There was little difference in the strength of the bones of the hens, undoubtedly because the bones were mature before the feeding began, and were little affected by the feeding. We find, however, that the bones of the chickens fed on nitrogenous food were almost fifty per cent. (49.6) stronger than those fed carbonaceous food.
The difference in the composition of the flesh, as shown by the analysis of Mr. W.P. Cutter, is given below:
| Hens. | Chickens. | |||
| Lot I. | Lot II. | Lot I. | Lot II. | |
| Nitrogenous. | Carbonaceous. | Nitrogenous. | Carbonaceous. | |
| lb. | lb. | lb. | lb. | |
| Albuminoids. | 43.81 | 25.13 | 52.00 | 30.06 |
| Fat. | 12.59 | 20.76 | 5.54 | 11.34 |
The flesh of each group was submitted to a number of persons for a cooking test, and the almost unanimous verdict was that the flesh of the fowls fed a nitrogenous ration was darker colored, more succulent, more tender, and better flavored, though on this last there was some difference of opinion.
So far as it is warrantable to draw any conclusions from a single experiment of this kind, it would seem that:
Chickens fed on an exclusive corn diet will not make a satisfactory development, particularly of feathers.
The bones of chickens fed upon a nitrogenous ration are fifty per cent. stronger than those fed upon a carbonaceous ration.
Hens fed on a nitrogenous ration lay many more eggs but of smaller size and poorer quality than those fed exclusively on corn.
Hens fed on corn, while not suffering in general health, become sluggish, deposit large masses of fat on the internal organs, and lay a few eggs of large size and excellent quality.
The flesh of nitrogenous fed fowls contains more albuminoids and less fat than those fed on a carbonaceous ration, and is darker colored, juicier and tenderer.
I.P. ROBERTS, Director.
My attention has been called a number of times to the unsatisfactory records and directions concerning the grafting of herbaceous plants. There appears to have been very little attention given to the subject, and the scant discussions of it are mostly copied from one author to another. A few years ago I made some attempts at herbaceous grafting, but it was not until last winter that experiments were seriously undertaken. The work was put in the hands of J.R. Lochary as a subject for a graduating thesis.
The experiments were undertaken primarily for the purpose of learning the best methods of grafting herbs, but a secondary and more important object was the study of the reciprocal influences of stock and cion, particularly in relation to variegation and coloration. This second feature of the work is still under way, in one form or another, and we hope for definite results in a few years. As a matter of immediate advantage, however, herbaceous grafting has its uses, particularly in securing different kinds of foliage and flowers upon the same plant. There is no difficulty in growing a half dozen kinds or colors, on geraniums, chrysanthemums, or other plants from one stock of the respective species.
Six hundred grafts were made in our trials last winter. It was found that the wood must be somewhat hardened to secure best results. The very soft and flabby shoots are likely to be injured in the operation of grafting, and union does not take place readily. Vigorous coleus stocks, three months old, gave best results if cut to within two or three inches of the pot and all or nearly all the leaves removed from the stump. Geraniums, being harder in wood, made good unions at almost any place except on the soft growing points. The stock must not have ceased growth, however. Most of the leaves should be kept down on the stock. Cions an inch or two long were usually taken from firm growing tips, in essentially the same manner as in the making of cuttings. Sometimes an eye of the old wood was used, and in most cases union took place and a new shoot arose from the bud. The leaves were usually partly removed from the cion.
Various styles of grafting were employed, of which the common cleft and the veneer or side graft were perhaps the most satisfactory. In most instances it was only necessary to bind the parts together snugly with bass or raffia. In some soft wooded plants, like coleus, a covering of common grafting wax over the bandage was an advantage, probably because it prevented the drying out of the parts. In some cases, however, wax injured the tissues where it overreached the bandage. Sphagnum moss was used in many cases tied in a small mass about the union, but unless the parts were well bandaged the cion sent roots into the moss and did not unite, and in no case did moss appear to possess decided advantages. Best results were obtained by placing the plants at once in a propagating frame, where a damp and confined atmosphere could be obtained. In some plants, successful unions were made in the open greenhouse, but they were placed in shade and kept sprinkled for a day after the grafts were made. The operation should always be performed quickly to prevent flagging of the cions. Or, if the cions cannot be used at once, they may be thrust into sand or moss in the same manner as cuttings, and kept for several days. In one series, tomato and potato cuttings, which had flagged in the cutting bed, revived when grafted. And cuttings which had been transported in the mail for three days grew readily, but they were in good condition when received. The mealy bugs were particularly troublesome upon these grafted plants, for they delighted to crawl under the bandages and suck the juices from the wounded surfaces.
Although it is foreign to the purpose of this note, it may be worth while to mention a few of the plants upon which the experiments were made. Sections were taken of many of the grafts and microscopic examinations made to determine the extent of cell union. Coleuses of many kinds were used, with uniform success, and the cions of some of them were vigorous a year after being set. Even iresine (better known as Achyranthes Verschaffeltii) united with coleus and grew for a time. Zonale geraniums bloomed upon the common rose geranium. Tomatoes upon potatoes and potatoes upon tomatoes grew well and were transplanted to the open ground, where they grew, flowered and fruited until killed by frost. The tomato-on-potato plants bore good tomatoes above and good potatoes beneath, even though no sprouts from the potato stock were allowed to grow. Peppers united with tomatoes and tomatoes united with peppers. Egg plants, tomatoes and peppers grew upon the European husk tomato or alkekengi (Physalis Alkekengi). Peppers and egg plants united with each other reciprocally. A coleus cion was placed upon a tomato plant and was simply bound with raffia. The cion remained green and healthy, and at the end of forty-eight days the bandage was removed, but it was found that no union had taken place. Ageratums united upon each other with difficulty. Chrysanthemums united readily. A bean plant, bearing two partially grown beans, chanced to grow in a chrysanthemum pot. The stem bearing the pods was inarched into the chrysanthemum. Union took place readily, but the beans turned yellow and died. Pumpkin vines united with squash vines, cucumbers with cucumbers, muskmelons with watermelons, and muskmelons, watermelons and cucumbers with the wild cucumber or balsam apple (Echinocystis lobata).
Another interesting feature of the work was the grafting of one fruit upon another, as a tomato fruit upon a tomato fruit or a cucumber upon another cucumber. This work is still under progress and it promises some interesting results in a new and unexpected direction, reports of which may be expected later.—Cornell Station Bulletin.
This article is condensed by permission from a thesis prepared for the degree of Bachelor of Science in Agriculture, by James Edward Rice, a graduate of the class of 1890. The work was planned and wholly carried out in the most careful manner by Mr. Rice under the immediate supervision of the Director. The results have been thought worthy of publication in the Cornell Station Bulletin.
The Michigan State Board of Health recently took Health Officer Davis, of Close Village, to task for failing to send in his weekly reports. His reply was unique. He says: "There has not been enough sickness here the last two or three years to do much good. The physicians find time to go to Milwaukee on excursions, serve as jurors in justice courts, sit around on drygoods boxes, and beg tobacco, chew gum, and swap lies. A few sporadic cases of measles have existed, but they were treated mostly by old women, and no deaths occurred. There was an undertaker in the village, but he is now in the State prison. It is hoped and expected when green truck gets around, melons plenty, and cucumbers in abundance, that something may revive business. If it does, I will let you know."
Contained in SCIENTIFIC AMERICAN SUPPLEMENT during the past ten years, sent free of charge to any address. MUNN & CO., 361 Broadway, New York.
$2.50 a Year. Single Copies, 25 cts.
This is a Special Edition of the SCIENTIFIC AMERICAN, issued monthly—on the first day of the month. Each number contains about forty large quarto pages, equal to about two hundred ordinary book pages, forming, practically, a large and splendid Magazine of Architecture, richly adorned with elegant plates in colors and with fine engravings, illustrating the most interesting examples of modern Architectural Construction and allied subjects.
A special feature is the presentation in each number of a variety of the latest and best plans for private residences, city and country, including those of very moderate cost as well as the more expensive. Drawings in perspective and in color are given, together with full Plans, Specifications, Costs, Bills of Estimate, and Sheets of Details.
No other building paper contains so many plans, details, and specifications regularly presented as the SCIENTIFIC AMERICAN. Hundreds of dwellings have already been erected on the various plans we have issued during the past year, and many others are in process of construction.
Architects, Builders, and Owners will find this work valuable in furnishing fresh and useful suggestions. All who contemplate building or improving homes, or erecting structures of any kind, have before them in this work an almost endless series of the latest and best examples from which to make selections, thus saving time and money.
Many other subjects, including Sewerage, Piping, Lighting, Warming, Ventilating, Decorating, Laying out of Grounds, etc., are illustrated. An extensive Compendium of Manufacturers' Announcements is also given, in which the most reliable and approved Building Materials, Goods, Machines, Tools, and Appliances are described and illustrated, with addresses of the makers, etc.
The fullness, richness, cheapness, and convenience of this work have won for it the Largest Circulation of any Architectural publication in the world.
A Catalogue of valuable books on Architecture, Building, Carpentry, Masonry, Heating, Warming, Lighting, Ventilation, and all branches of industry pertaining to the art of Building, is supplied free of charge, sent to any address.
MUNN& CO., Publishers,
361 Broadway, New York.
In connection with the publication of the BUILDING EDITION of the SCIENTIFIC AMERICAN, Messrs. Munn & Co. furnish plans and specifications for buildings of every kind, including Churches, Schools, Stores, Dwellings, Carriage Houses, Barns, etc.
In this work they are assisted by able and experienced architects. Full plans, details, and specifications for the various buildings illustrated in this paper can be supplied.
Those who contemplate building, or who wish to alter, improve, extend, or add to existing buildings, whether wings, porches, bay windows, or attic rooms, are invited to communicate with the undersigned. Our work extends to all parts of the country. Estimates, plans, and drawings promptly prepared. Terms moderate. Address
MUNN & CO., 361 BROADWAY, NEW YORK.
PUBLISHED WEEKLY.
Terms of Subscription, $5 a year.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of THE SUPPLEMENT, from the commencement, January I, 1876, can be had. Price, 10 cents each.
All the back volumes of THE SUPPLEMENT can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50 stitched in paper, or $3.50 bound in stiff covers.
COMBINED RATES.—One copy of SCIENTIFIC AMERICAN and one copy of SCIENTIFIC AMERICAN SUPPLEMENT, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
361 Broadway, New York, N.Y..
Manufacturers, Agriculturists, Chemists, Engineers, Mechanics, Builders, men of leisure, and professional men, of all classes, need good books in the line of their respective callings. Our post office department permits the transmission of books through the mails at very small cost. A comprehensive catalogue of useful books by different authors, on more than fifty different subjects, has recently been published, for free circulation, at the office of this paper. Subjects classified with names of author. Persons desiring a copy have only to ask for it, and it will be mailed to them. Address,
MUNN & CO., 361 Broadway, New York.
In connection with the Scientific American, Messrs. MUNN & Co. are solicitors of American and Foreign Patents, have had 42 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to MUNN & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats, Trade Marks, their costs and how procured. Address
MUNN & CO.,
361 Broadway, New York.
Branch Office, 622 and 624 F St., Washington, D.C.